Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Linked Comprehension)|4 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Multiple Correct)|3 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Fill In The Blanks)|15 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Archives Subjective|10 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Ex 5.1 (Objective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY-Exercises (True/False)
- CI(2)O(6) is a mixed anhydride of HCIO(3) and HCIO(4).
Text Solution
|
- Sea weads are important source of bromine.
Text Solution
|
- Antichlor is a compound which removes chlorine form a material.
Text Solution
|
- HIF can be stronger in glass bottles.
Text Solution
|
- I(2) cannot liberate CI(2) form aqueous KCI but it can liberate CI(2) ...
Text Solution
|
- Conc. H(2)SO(4) cannot be used to prepared HBr form NaBr.
Text Solution
|
- HBr is stronger acid than HI because of hydrogen bonding.
Text Solution
|
- I(4)O(9) is a covalent compound.
Text Solution
|
- Halogen that is most easily reduced is flurine.
Text Solution
|
- Solid CI(2)O(6) is a covalnt compound.
Text Solution
|
- ICI(3) is an example psedohalogen.
Text Solution
|
- Iodine is used as an antisepitc.
Text Solution
|
- Tear gas is C CI(3).SO(2) .
Text Solution
|
- Chlorine is known as superhalogen.
Text Solution
|
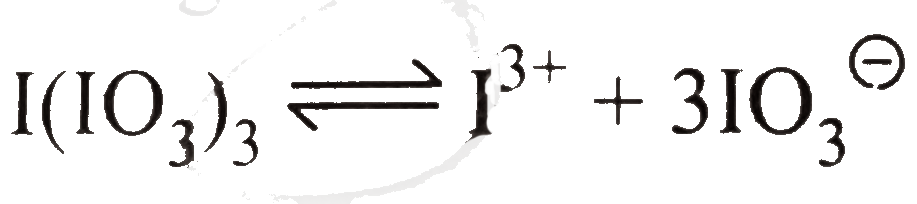 `I^(3+)+3IO_(3)^(Theta)`
`I^(3+)+3IO_(3)^(Theta)`