A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Exercises (Assertion And Reason)|12 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Exercises (Integer)|7 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|7 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise True/False (Subjective)|14 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 18 ELEMENTS - THE INERT GASES-Exercises (Single Correct)
- Electronegativety of an inert gas is
Text Solution
|
- Which has the same electronic configuration as of inert gas ?
Text Solution
|
- Which of the following noble gas is not present in atmosphere ?
Text Solution
|
- Which noble gas is more soluble in water ?
Text Solution
|
- XeF(6) on complete hydrolysis gives
Text Solution
|
- Xenon tetrafluoride has hybridisation and structure as
Text Solution
|
- Which noble gas has higher and least polarisability respectively ?
Text Solution
|
- Which is monoatomic?
Text Solution
|
- In the clathrates of xenon with water , the nature of bonding between ...
Text Solution
|
- Asthma patients use a mixture of …..for respiration
Text Solution
|
- The solubility of noble gases in water shown the order
Text Solution
|
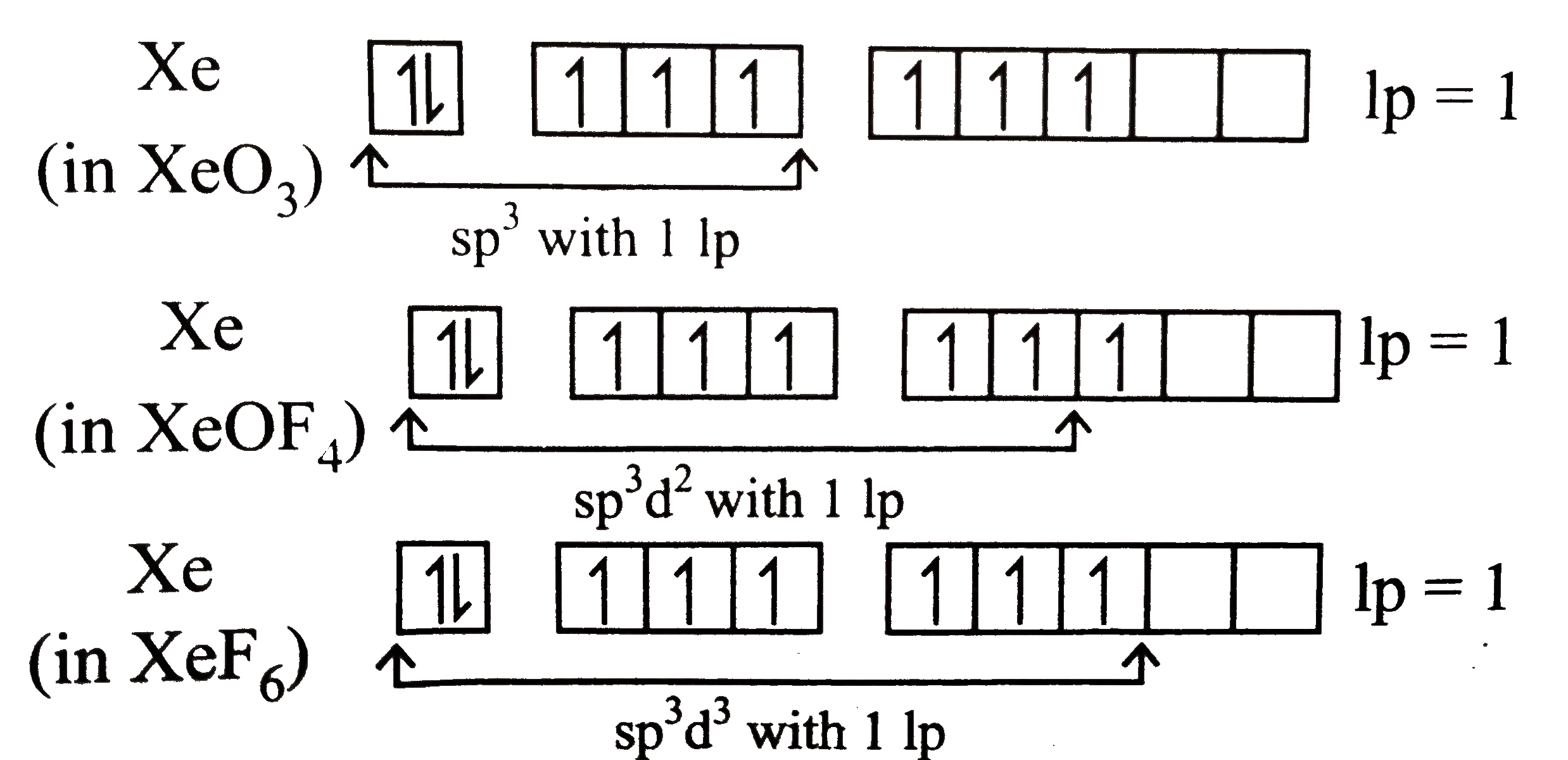
- Out of (i) XeO(3)(ii) XeOF(4) and (iii) XeF(6) the molecules having sa...
Text Solution
|
- Which is planar molecule ?
Text Solution
|
- Which of the following cannot he formed ?
Text Solution
|
- Which statement regarding He is incorrect?
Text Solution
|
- Which of the following is an explosive compound ?
Text Solution
|
- The idea which prompted bartlett to prepare first ever compound of no...
Text Solution
|
- Noble gases are also known as aerogens because
Text Solution
|
- The gaseous mixture used by deep sea divers for respiration is
Text Solution
|
- Percentage of argon in air is
Text Solution
|