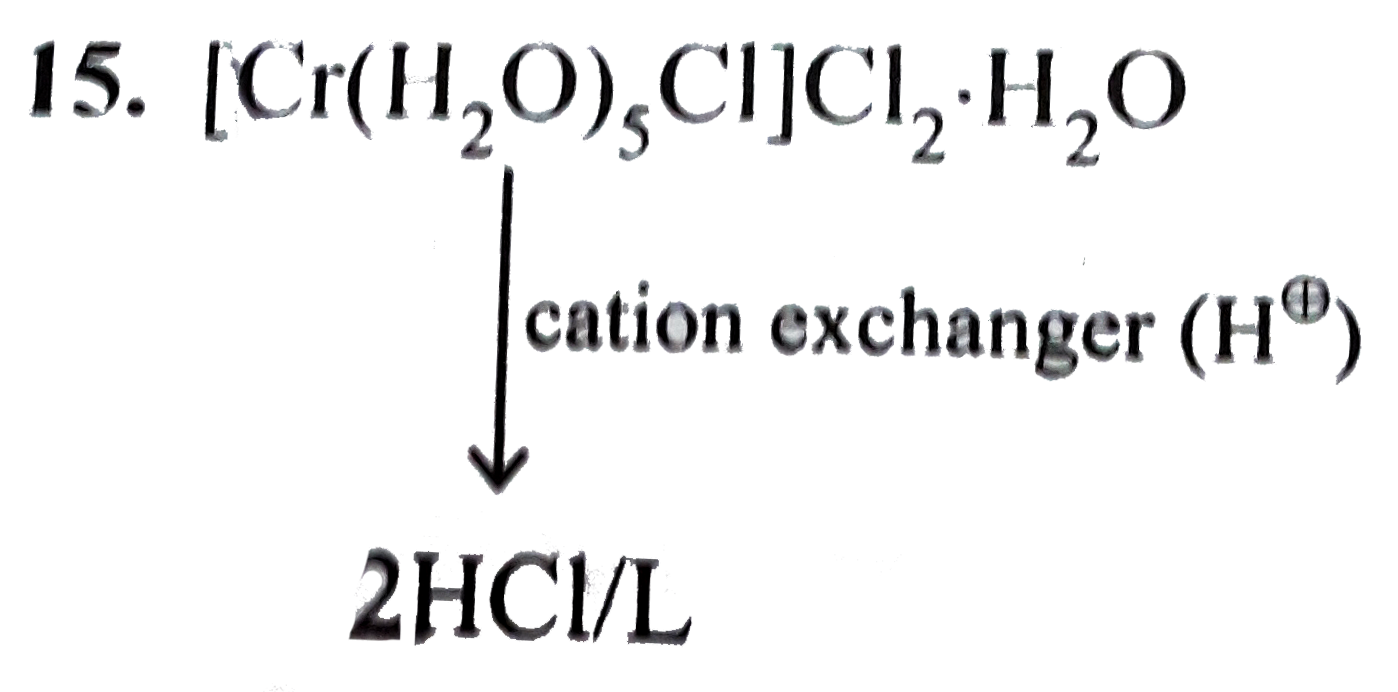Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Objective (Terminology)|16 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Objective (Isomerism)|12 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Subjective (Conductance In Coordination Compounds)|2 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-COORDINATION COMPOUNDS-Ex 7.1 Subjective (Isomerism In Coordination Compounds)
- how will you distinguish between the following pairs of isomers [Cr(NH...
Text Solution
|
- How many geometrical isomers are possible for the complex ion [Cr(NH(3...
Text Solution
|
- The complex M(C(4)O(4))CI(2)(NH(3))(2) forms two types of ionic colour...
Text Solution
|
- The compound Co(en)(2)(NO(2))(2)CI has been prepared in these isomeric...
Text Solution
|
- A solution containing 1 go of the complex [Cr(H(2)O)(5)CI]CI(2)H(2)O...
Text Solution
|
- A solution containing 2.675g of CoCI(3).6NH(3) was passed through a ca...
Text Solution
|