Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Objective (Isomerism)|12 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.2 Subjective|4 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Subjective (Isomerism In Coordination Compounds)|6 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-COORDINATION COMPOUNDS-Ex 7.1 Objective (Terminology)
- The oxidation number of Co in [Co(en)(3)](2)(SO(4))(3) is +2 +1 ...
Text Solution
|
- The IUPAC name of the coordination compound Na(3)[Ag(S(2)O(3))(2)] is ...
Text Solution
|
- The IUPAC name of the coordination compound [CuCI(2)(CH(3)NH(2))(2)] i...
Text Solution
|
- The IUPAC name for [AI(OH)(H(2)O)(5)]^(2+) is (a) Pentahyroaluminium...
Text Solution
|
- The IUPA name of [Pt(Br)(CI)(NH(3))(3)(NO(2))]CI is (a) Triamminechl...
Text Solution
|
- The oxidation number of Cr in [Cr(C(6)H(2))(2] is 0 (b) +2 (c ) +3...
Text Solution
|
- Which of the following has five donor (coordinating) sites? (a) Ethy...
Text Solution
|
- Which of the following is not chelating agent (a) Thiosulphate (b)...
Text Solution
|
- The solution of AgBr in presence of large excess of NH(3) contains mai...
Text Solution
|
- Which of the following species is not expected to be a ligand overse...
Text Solution
|
- The number of donor sites in dimethy1 glyoxime glycinato diethylene tr...
Text Solution
|
- Which of the following is a double salt ?
Text Solution
|
- When potash alum is dissolved in water the total number of ions produc...
Text Solution
|
- Which of the following statements is correct with regard to complex io...
Text Solution
|
- How many mloes of of AgCI would be obtained when 100mL of 0.1 M Co(NH...
Text Solution
|
- 0.001 mol of Cr(NH(3))(5)(NO(3))(SO(4)) was passed through a cation ex...
Text Solution
|
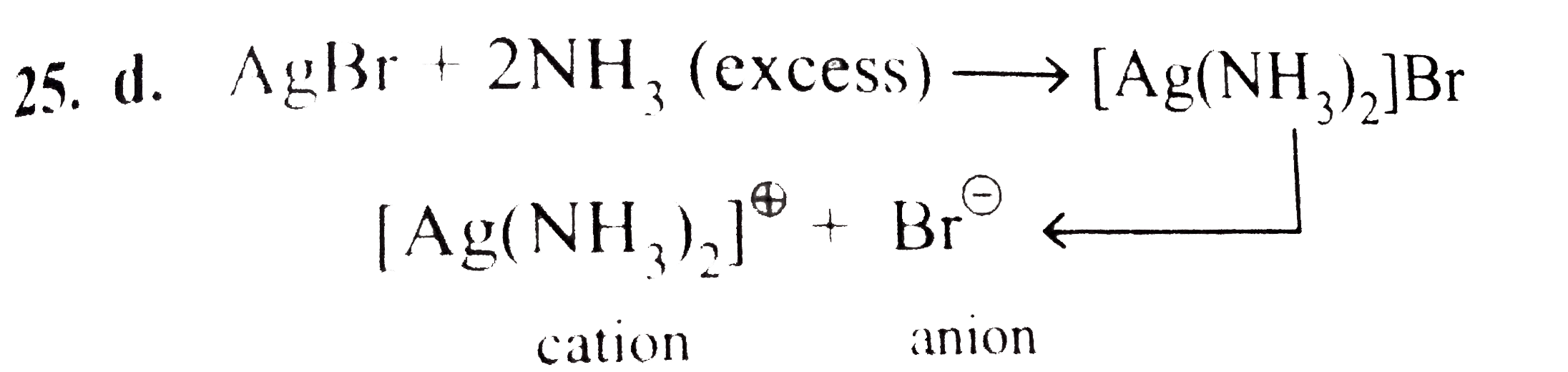 .
.