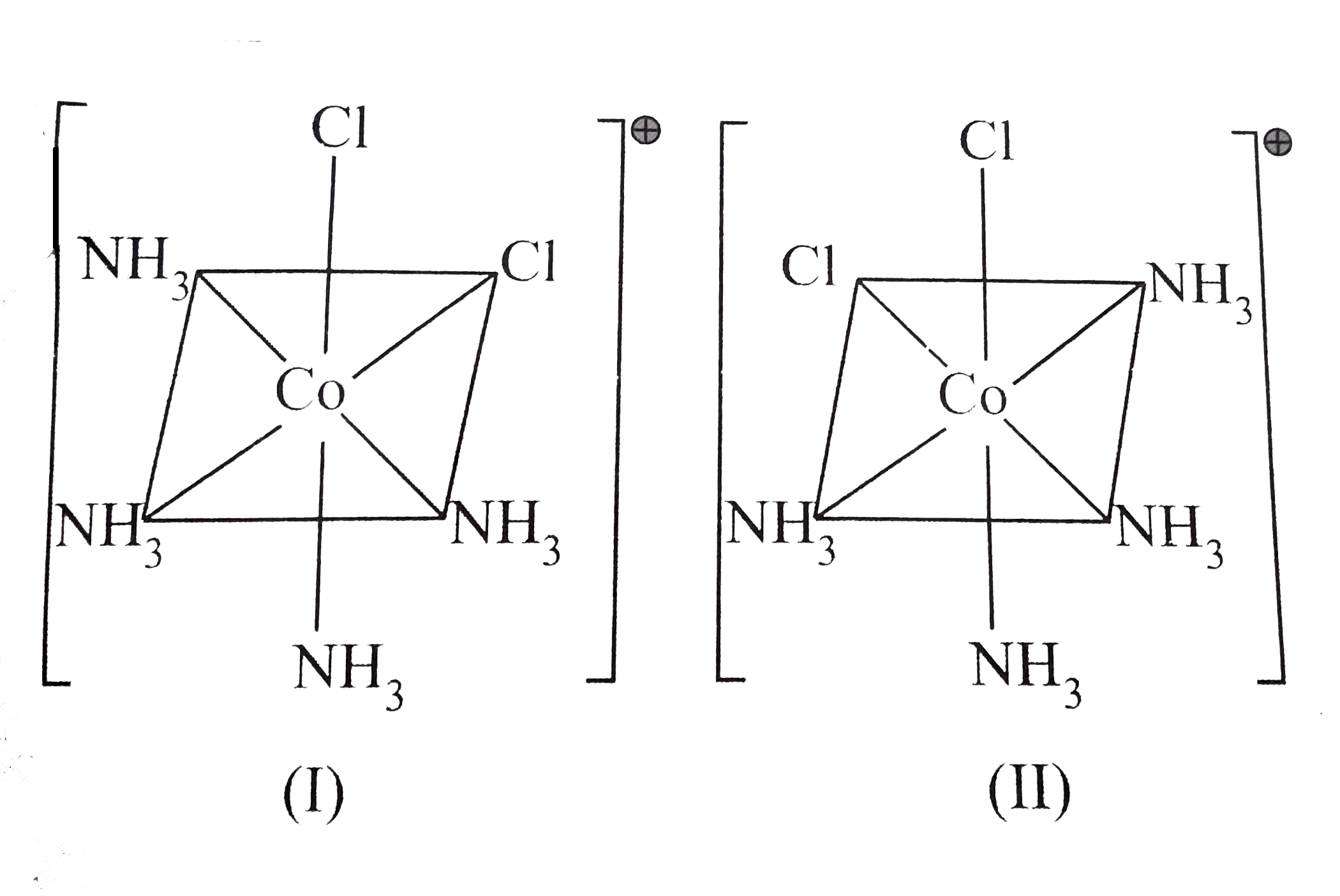
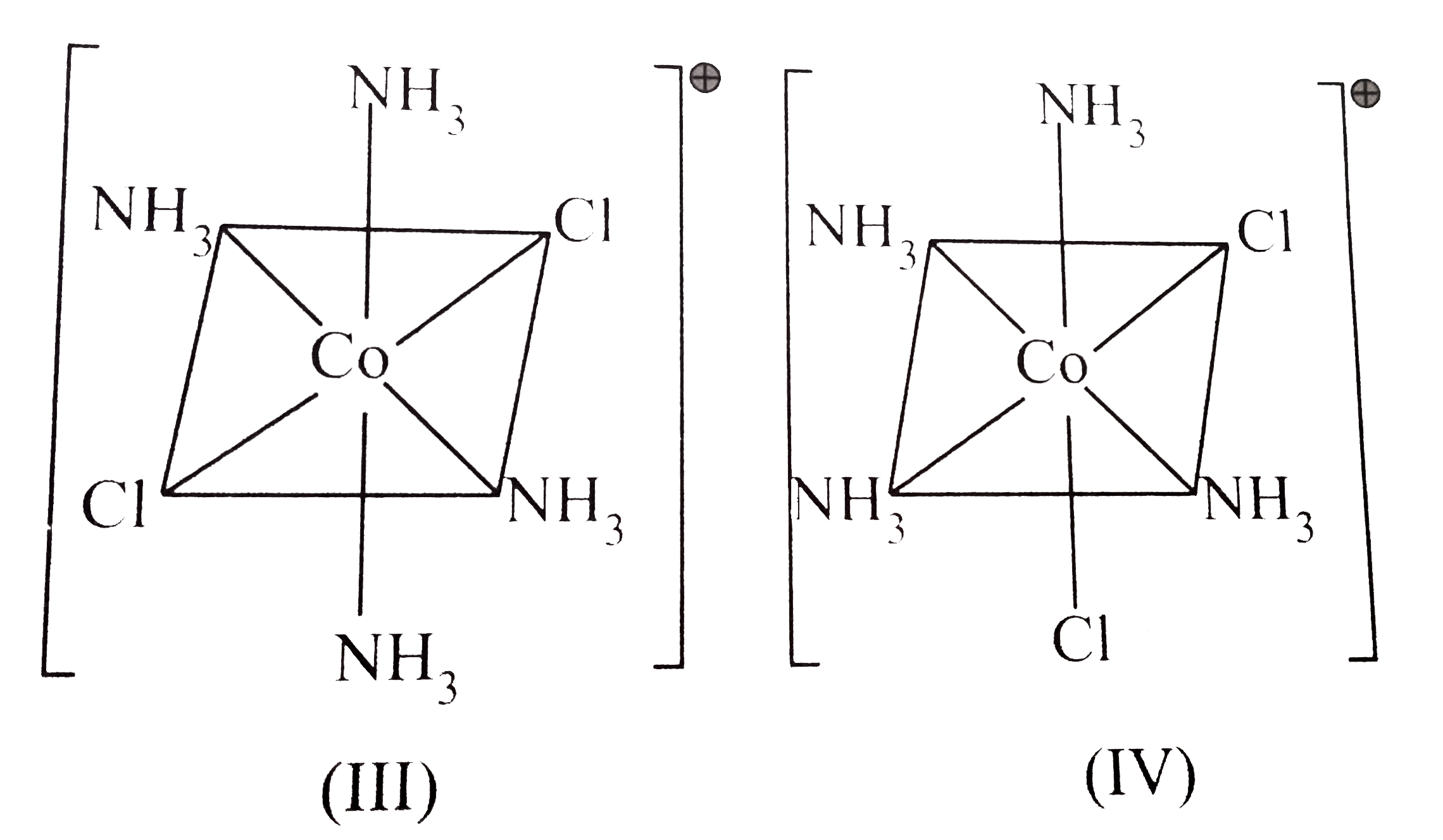
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.2 Subjective|4 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.2 Objective|8 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Ex 7.1 Objective (Terminology)|16 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-COORDINATION COMPOUNDS-Ex 7.1 Objective (Isomerism)
- Which of the following coordination compounds exhibits ionisation isom...
Text Solution
|
- Which of the following complex compounds exhibits cis-trans isomerism ...
Text Solution
|
- How many geometrical isomers are possible for the square planar comple...
Text Solution
|
- Consider the following spatial arrangements of the octahedral complex ...
Text Solution
|
- Which of the following pairs of structures represent facial and meridi...
Text Solution
|
- Which would exhibit coordination isomerism [Cr(NH(3))(6)][Co(CN)(6...
Text Solution
|
- Which would exhibit ionisation isomerism (a) p[Co(NH(3))(6)][(C(2)O(...
Text Solution
|
- The water -soluble complex among the following is (a) [Ni(HDMG)(2)] ...
Text Solution
|
- Arrage the following optical activity possible in (a) [Co(H(2)O)(2)(...
Text Solution
|
- When an excess of ammonia solution is added to CuSO(4) which solution ...
Text Solution
|
- Copper sulphate solution reacts with KCN to give (a) Cu(CN)(2) (b)...
Text Solution
|
- The ionisation isomer of [Co(H(2)O)(4)CI(2)NO (a) [Co(H(2)O)(4)(NO...
Text Solution
|