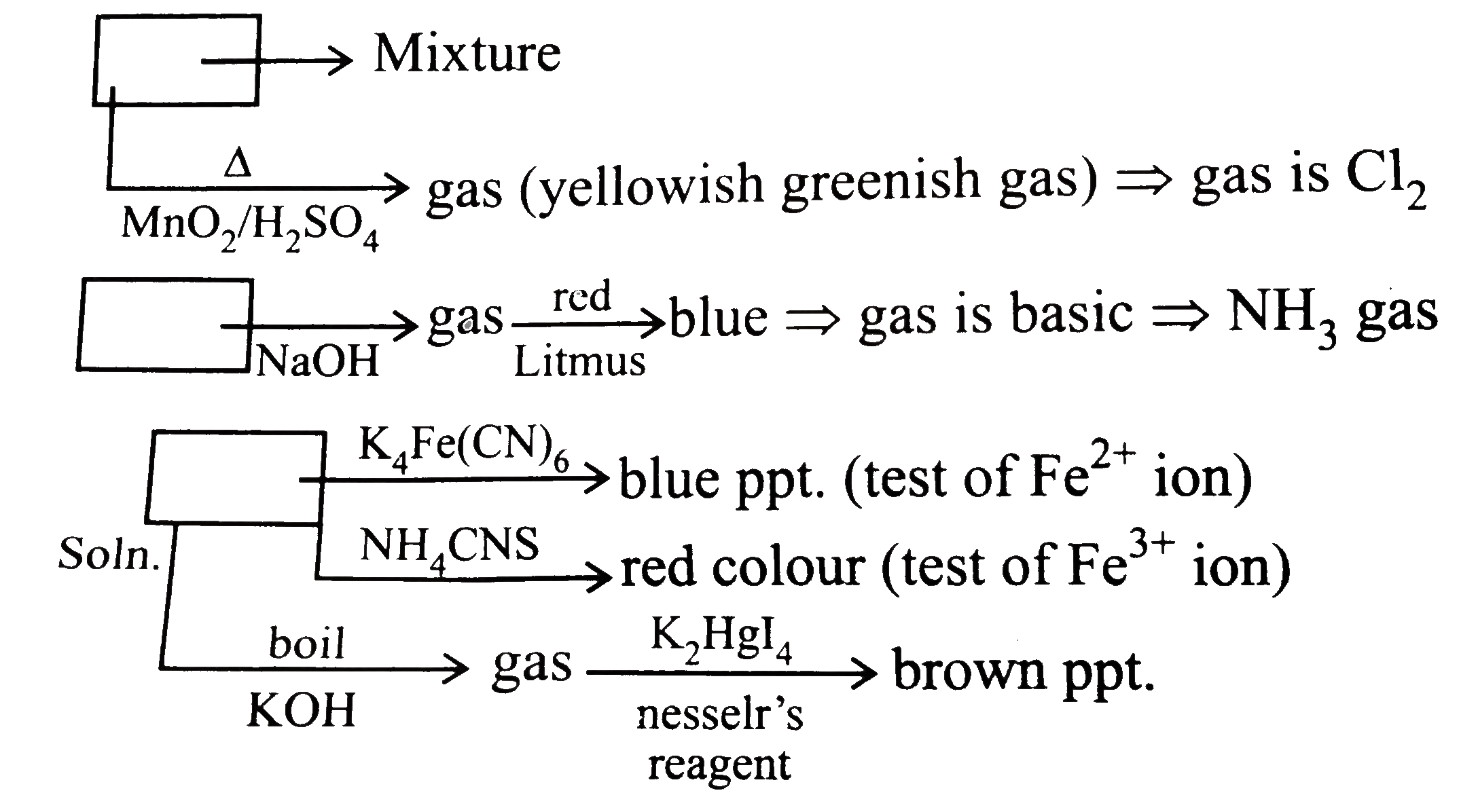Text Solution
Verified by Experts
Topper's Solved these Questions
QUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Ex 8.1|14 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Ex 8.2|79 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY|Exercise Exercises Archives (True/False)|2 VideosP-BLOCK GROUP 18 ELEMENTS - THE INERT GASES
CENGAGE CHEMISTRY|Exercise Ex 5.1 (Objective)|14 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|3 Videos
CENGAGE CHEMISTRY-QUALITATIVE INORGANIC SALT ANALYSIS-Exercises Archives (Subjective)
- Menion the products formed in the following i. Zine oxide is trea...
Text Solution
|
- Write the balanced equition for the following ''Potassium permangan...
Text Solution
|
- A mixture of two salt was treated as follows : i. The mixture was ...
Text Solution
|
- Write the balancexd chemical equation for the following i. Silver...
Text Solution
|
- The gas librated , on heating a mixture of two salts with NaOH giv...
Text Solution
|
- Give reacts in one two sentence for the followin g ''The hydroxide ...
Text Solution
|
- In the fopllowing reaction , identify the compound / rteaction co...
Text Solution
|
- A light bluish-green crystaline compound responds to the following t...
Text Solution
|
- The acdic aqueous solution of ferrous ion forms a brown complex i...
Text Solution
|
- An orange solid A on heating gave qa green residue B, a colourless g...
Text Solution
|
- A scalet compound A is treated with concenbtrated HNO(3)to gave a ch...
Text Solution
|
- The gradiual addition of KJ solution to Bi(NO(3))(3) solution identif...
Text Solution
|
- Calcium burns in nitrogen to produce a white powder which dissolv...
Text Solution
|
- A colourless inorganic salt A decomposes completely as about 250^(@)C...
Text Solution
|
- Element A burns in nitrogen to give an ions compound B reacts with ...
Text Solution
|
- During the quation analysis of a mixture containing Cu^(2+) and Zn^...
Text Solution
|
- An aqueous solution containing 1 mol of HgI(2) and 2 mol of Nal is o...
Text Solution
|
- A white solid is either Na(2)O or Na(2)O(2). A piece of red litmus pa...
Text Solution
|
- Write theh chemical reaction associated with the ''brown ring test''
Text Solution
|
- An aqueous blue colour solution of a transition metal sulphate rea...
Text Solution
|