Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Concept|16 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Comprehension|20 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Example|18 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GENERAL ORGANIC CHEMISTRY-Subjective
- Give the decreasing order of acidic character of the following : (a...
Text Solution
|
- Give the decreasing order of basic character at a,b,c,d in the followi...
Text Solution
|
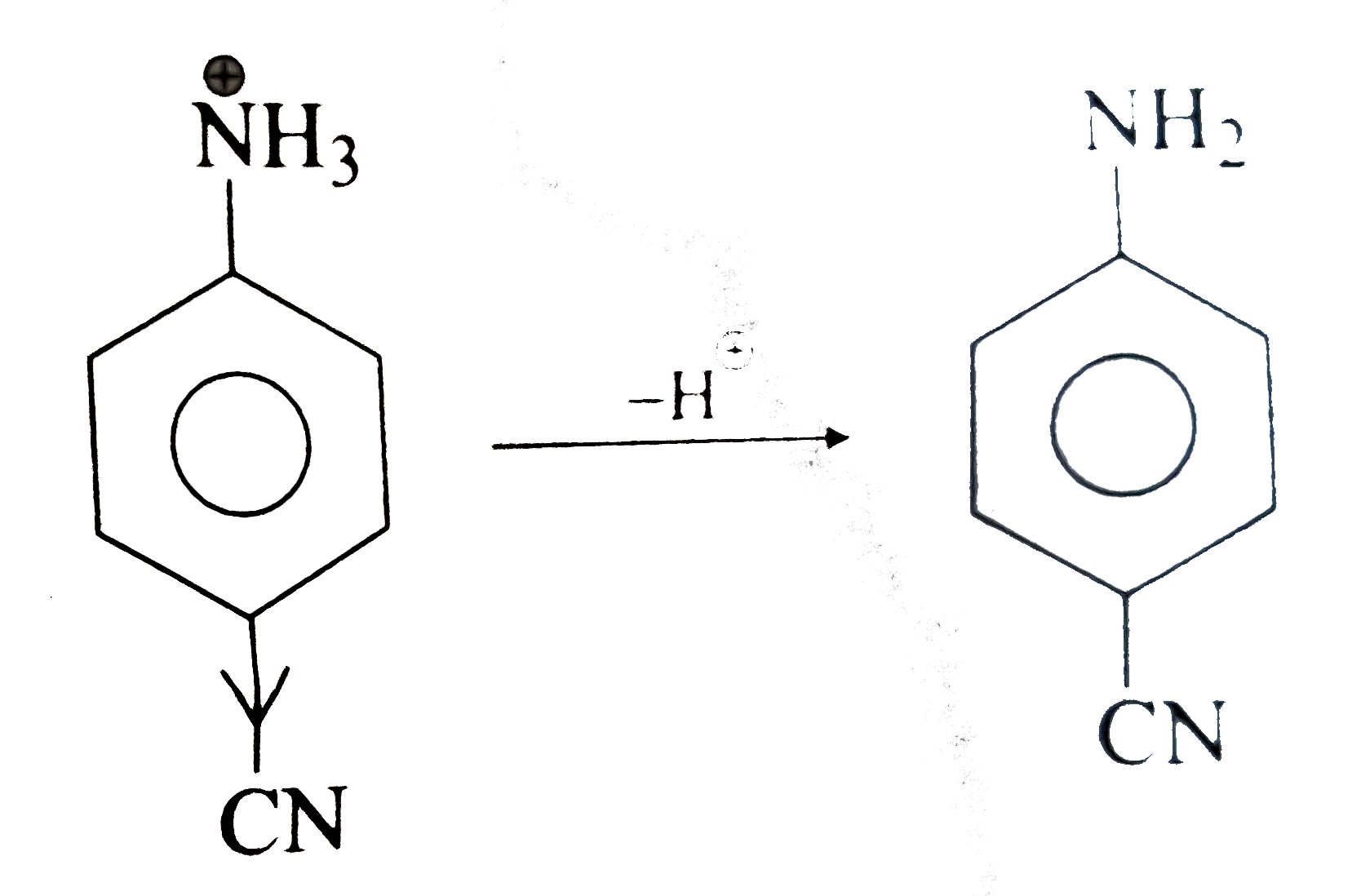
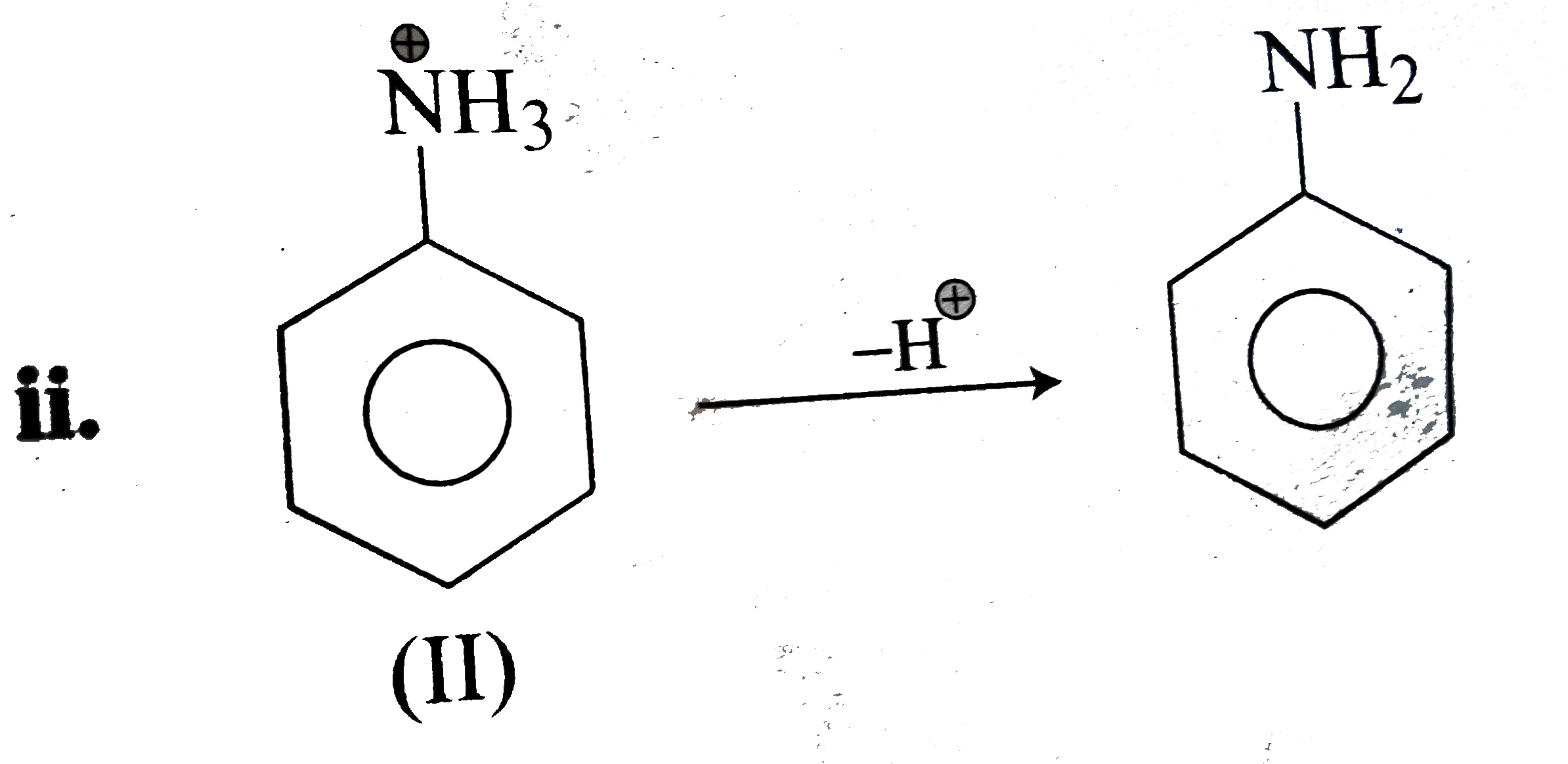
- Why cyanoanilinium ion (C6H4(CN) overset (oplus) N H3) (I) is a strong...
Text Solution
|
- Give the order of acidic character, o-,p-, and m-cyanoanilinium ions.
Text Solution
|
- Give the order of acidic character of the following : (i) (a) p-Nit...
Text Solution
|
- Give the order of the stabilities of the following : (i) (a) PhCH2^(...
Text Solution
|
- Why pyridine is a much weaker base than aliphatic amines ?
Text Solution
|
- Give the decreasing order of the stabilities of the following free rad...
Text Solution
|
- Give the decreasing order of acidic strength of the following : (a) ...
Text Solution
|
- Which is a stronger base towards a proton overset (Ө) P H 2 or overset...
Text Solution
|
- Give the decreasing order of basic strength of the following : (a) N...
Text Solution
|