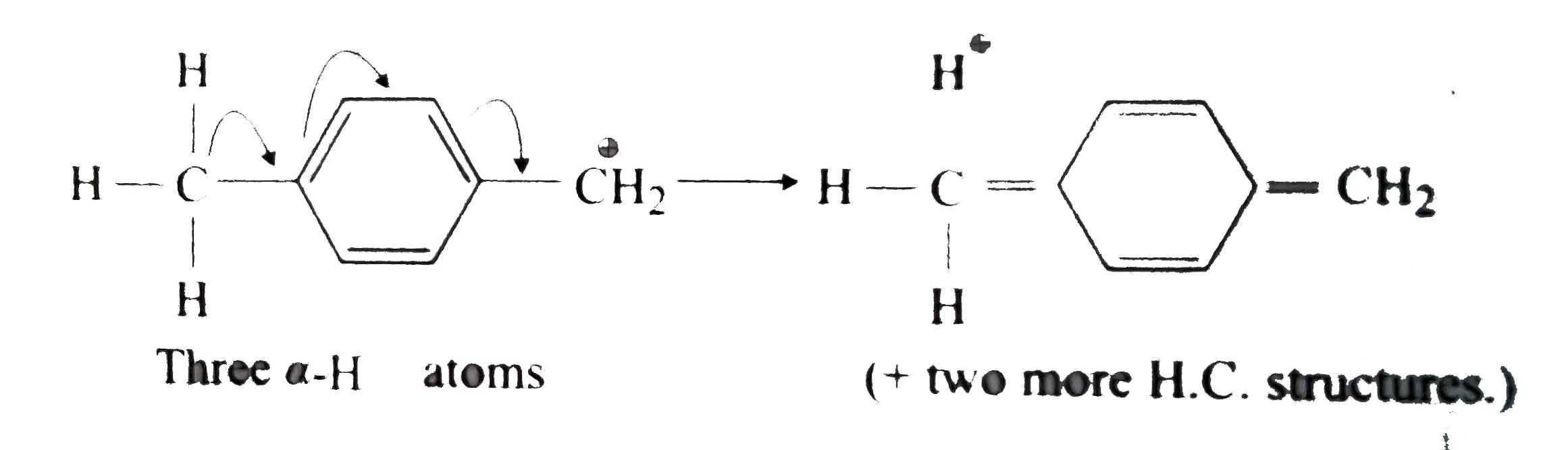
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives|12 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Fill in the blanks|4 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Single Correct|25 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive Type|3 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY|Exercise Subjective Archive (Subjective)|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GENERAL ORGANIC CHEMISTRY-Assertion-Reasoning
- Alkene A(Me2 C = CMe2) is more stable than alkene B(Et2 C = Cet2). B...
Text Solution
|
- Methylene has a sextet of overline e^, s. Methylene behaves as a nuc...
Text Solution
|
- Assertion(A):p-methyl benzyl carbocation (I) is more stable than benzy...
Text Solution
|
- Assertion(A):Me3 overset (-) C is more stable than overset (-) C H3. ...
Text Solution
|
- The pKa value of (I) is more than the pKa value to (II). Nonaromat...
Text Solution
|