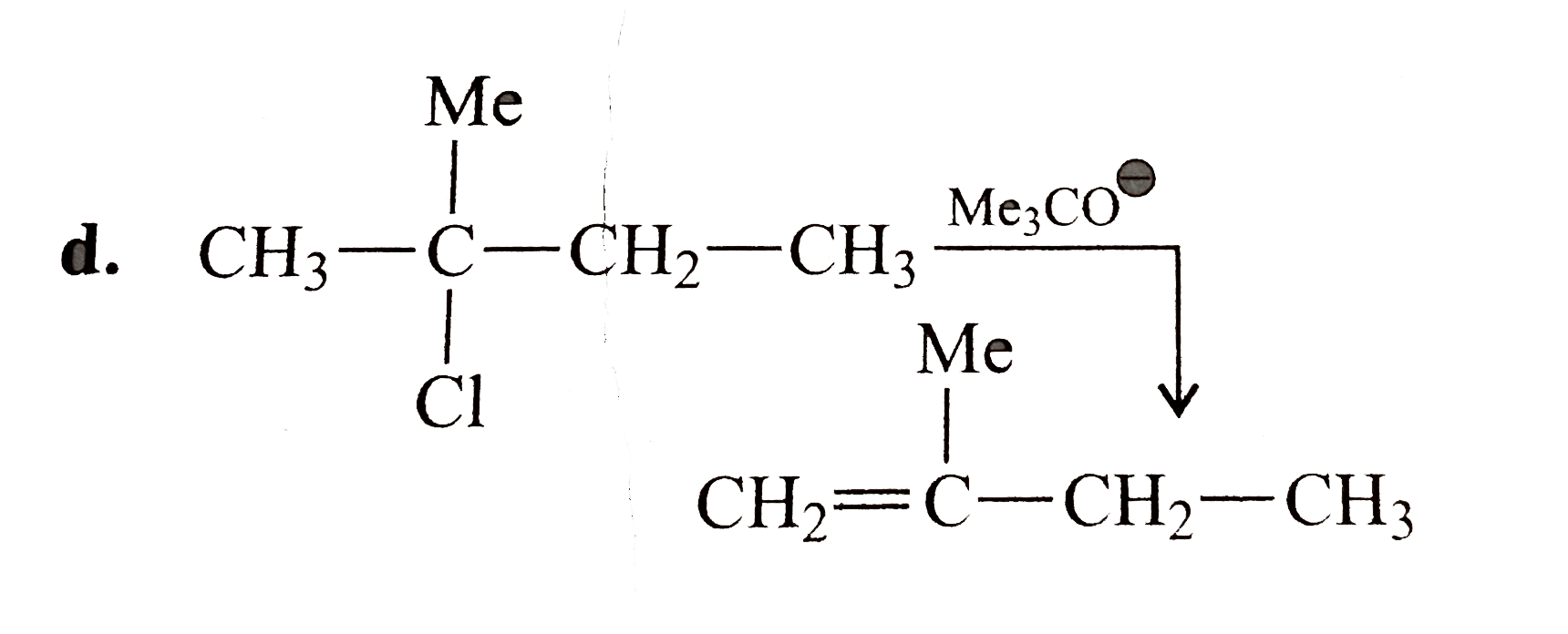Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Solved Example|33 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Subjective|19 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Chemical Equilibrium|72 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise Archives (Subjecive)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ORGANIC REACTION MECHANISM-Analytical and Descriptive
- (a) Compare the reactivity of Me3CO^(Ө)K^(oplus) and EtNH2 in E2 react...
Text Solution
|
- Draw the stereochemical structures of the products in the following re...
Text Solution
|
- Write down the structure of the stereoisomers formed when cis-2-butene...
Text Solution
|
- Optically active 2-iodo burtane on treatment with NaI in acetone gives...
Text Solution
|
- .
Text Solution
|
- The total number of basic groups in the following form of lysine is : ...
Text Solution
|
- Among the following, the total number of compound soluble in aqueous N...
Text Solution
|