Text Solution
Verified by Experts
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Subjective )|17 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|33 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Linked Comprehension)|1 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS-Solved Examples
- (A) and ( C) are isomers, (B) can be obtainedn by the products of the ...
Text Solution
|
- Two different G.R. (A) and (B) give the product (X) on reaction with ...
Text Solution
|
- . A sample of 0.42 gm os (A) with excess of MeMgBr gives 224 ml of C...
Text Solution
|
- Identify the structures of (A) to (D).
Text Solution
|
- Identify (A) to (F) and mark the C^(**) carbon in the entire scheme Ca...
Text Solution
|
- Identify (A) to ( C). .
Text Solution
|
- Identify (A) to ( E). .
Text Solution
|
- Identify (A) to ( E). .
Text Solution
|
- Identify (A) to (D). .
Text Solution
|
- How would the ratio of products change if. (i) R = Et (ii) (iii...
Text Solution
|
- Convert .
Text Solution
|
- Starting with (Propanol) and (ethanol). Prepare pent-2-ene using G...
Text Solution
|
- Complete the following reaction using G.R and any other compound. .
Text Solution
|
- Complete the following reactions : (a) (b) ( c) (d)
Text Solution
|
- Convert the following reactions by using G.R. (a)
Text Solution
|
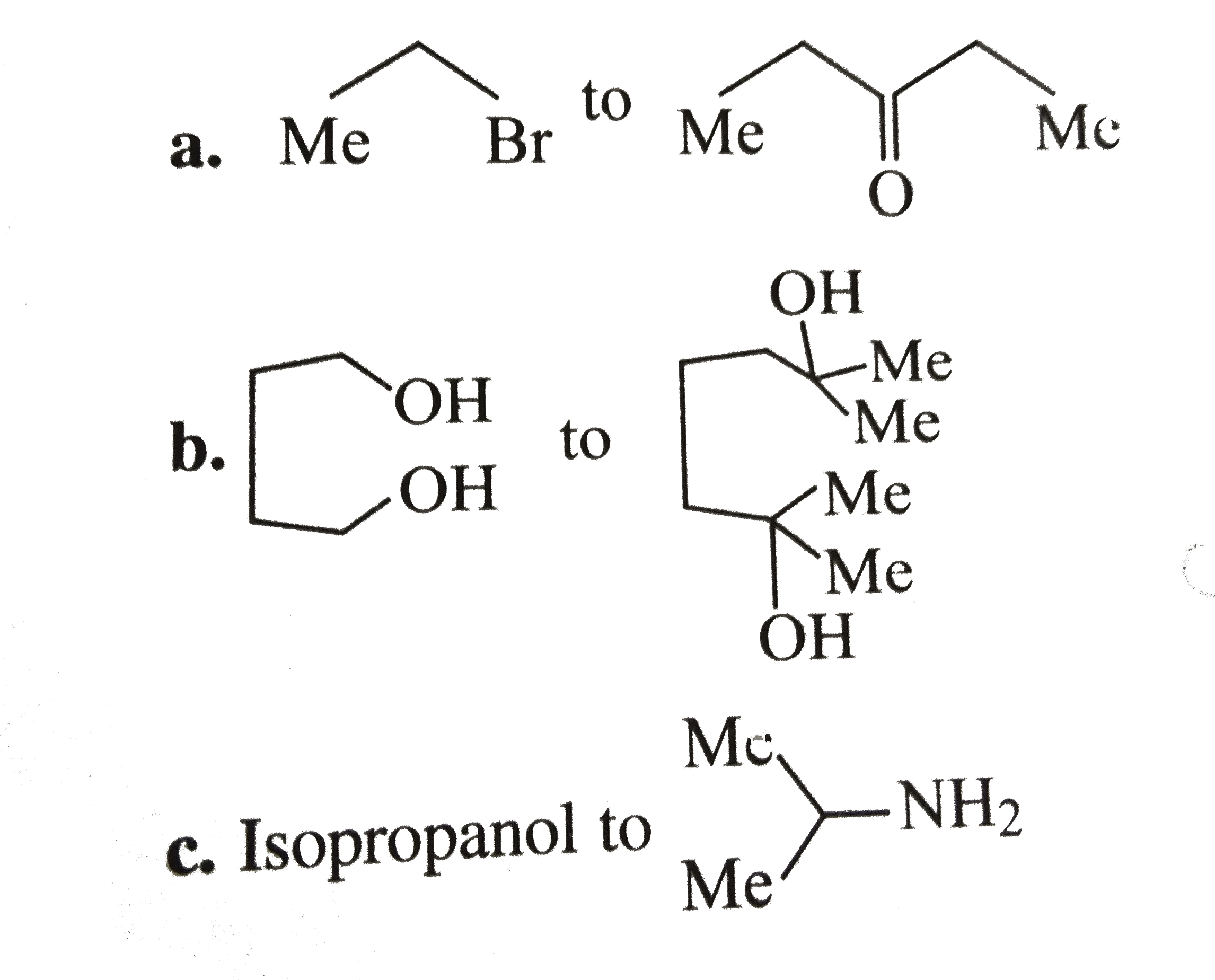

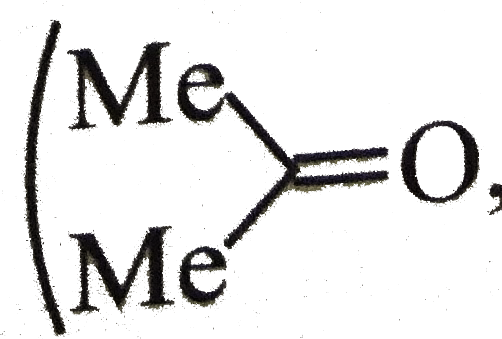 reacts with `G.R` and converts the reactant into dibromo compound and then into `d-G.R`. This `di-G.R` on reaction with acetone would give the desired product.
reacts with `G.R` and converts the reactant into dibromo compound and then into `d-G.R`. This `di-G.R` on reaction with acetone would give the desired product. 
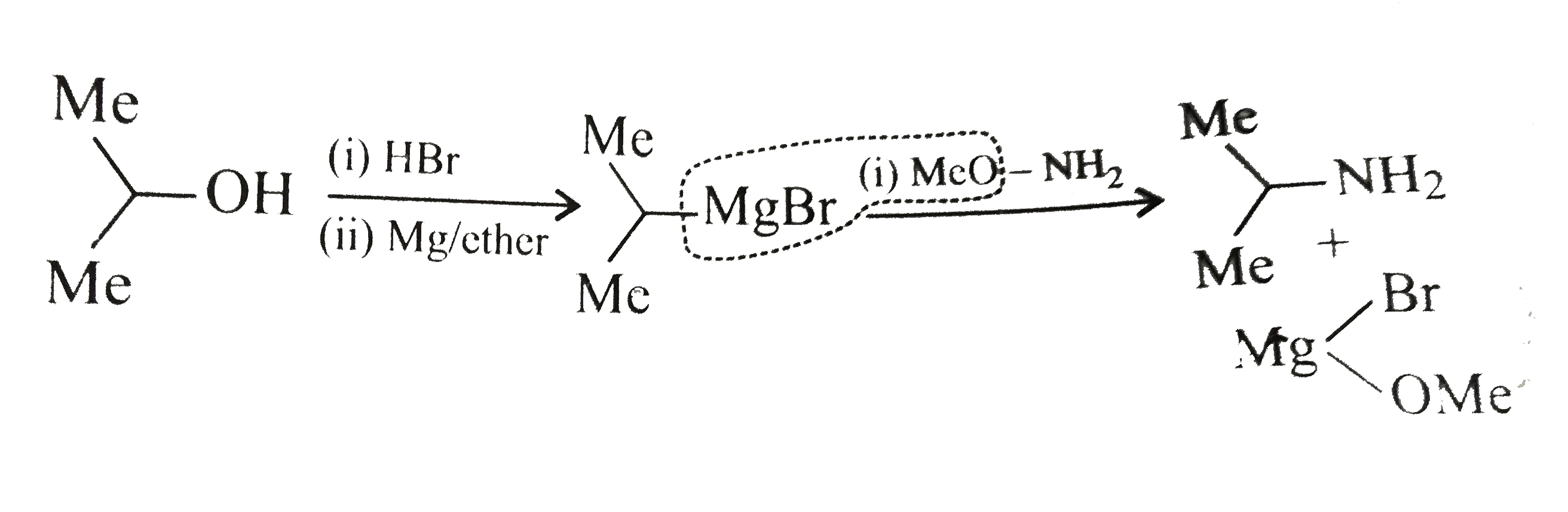 .
.