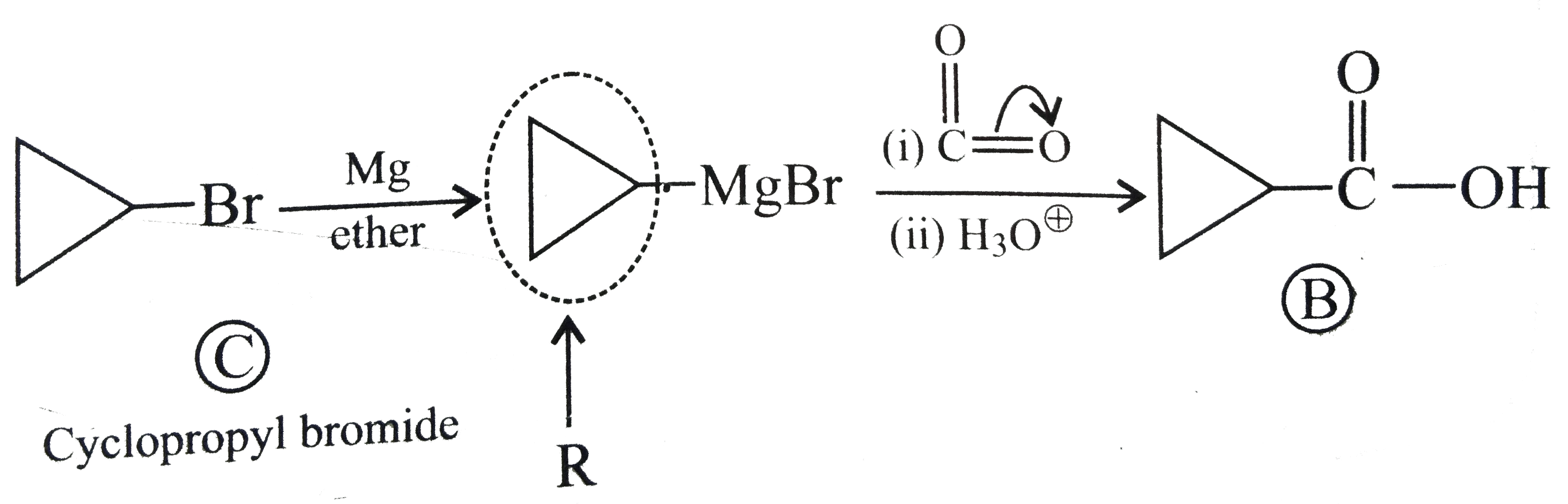Text Solution
Verified by Experts
Topper's Solved these Questions
GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Linked Comprehension)|1 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises Archives (True/False)|1 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Nuclear Chemistry (NCERT Exercise)|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS-Exercises Archives (Subjective)
- Write the structural formula of the main organic product formed when e...
Text Solution
|
- Compound X (molecular formula C5 H8 O) does not react appreciably with...
Text Solution
|
- Identify the major products in the following reaction. C6H5 COOH + M...
Text Solution
|
- In the following reactions, identify the compounds A, B, C and D. (i...
Text Solution
|
- 1,4-Pentadiene reacts with excess of HCl in the presence of benzoyl pe...
Text Solution
|
- A hydrocarbon A of the molecular formula C8 H10. On ozonolysis gives o...
Text Solution
|
- An ester A(C4 H8 O2), on treatement with excess of methyl magnesium bo...
Text Solution
|
- Cyclobutyl bromide on treatment with magnesium in dry ether forms an o...
Text Solution
|
- Identify Z + Y in the following synthetic scheme and write their struc...
Text Solution
|
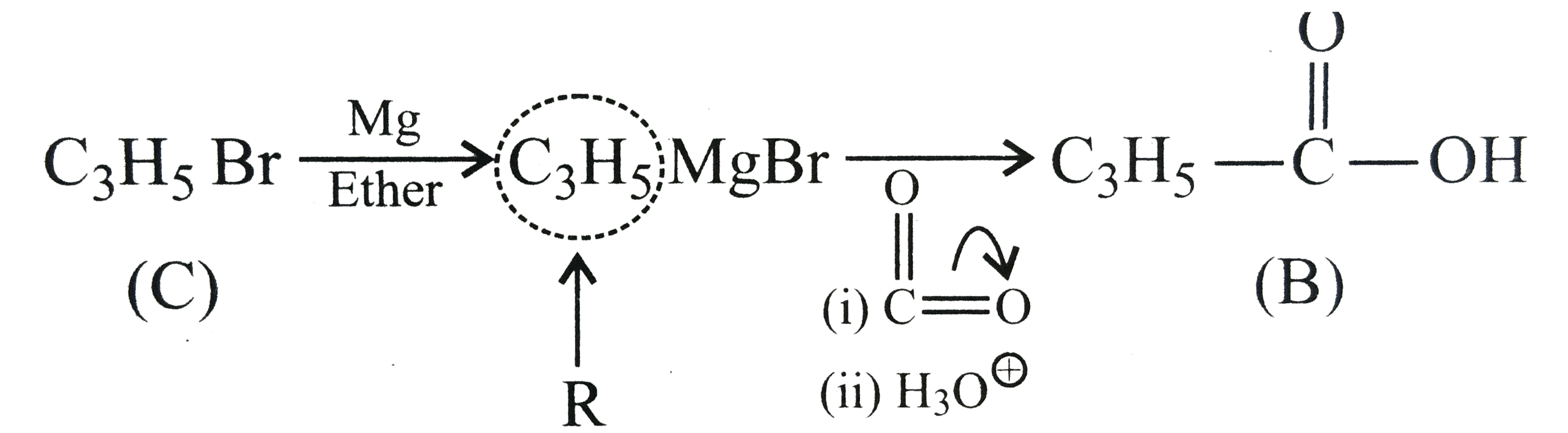
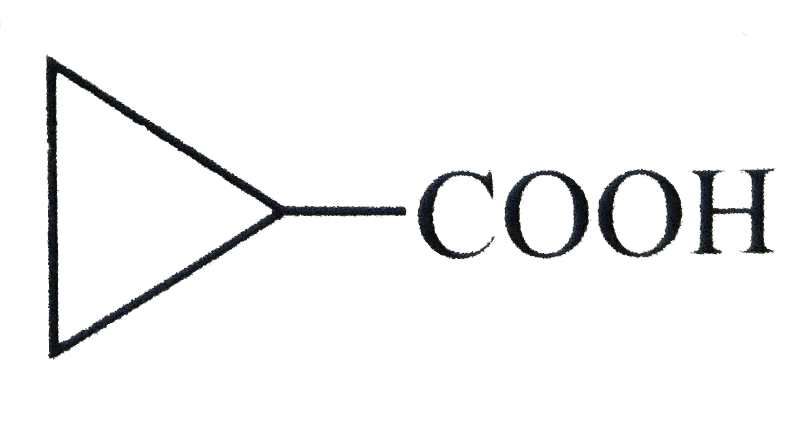
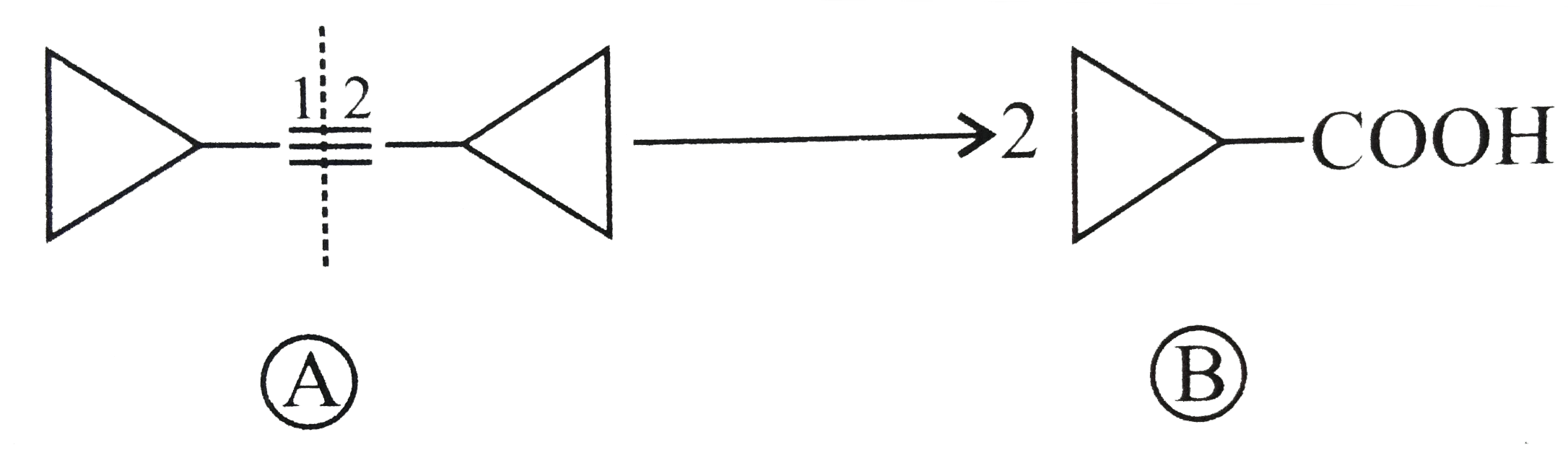 .
.