A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Single Correct)|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|35 VideosBIOMOLECULES, POLYMERS AND CHEMISTRY IN EVERYDAY LIFE
CENGAGE CHEMISTRY|Exercise QUESTION BANK|8 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Archives Subjective|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Exercises (Singlecorrect)
- Product ( C) is :
Text Solution
|
- and find out the reagent (A) from the following :
Text Solution
|
- The decreasing order of ease of hydrolysis of the following esters is ...
Text Solution
|
- The boiling points of ethyl acetate and CH3 OH, respectively, are 77^@...
Text Solution
|
- Carboxylic acid, although unreactive to alcohols, reacts in the presen...
Text Solution
|
- The major product ( C) in the reaction is : .
Text Solution
|
- Which of the following is the best method for the synthesis of ester (...
Text Solution
|
- Which of the following is the best method for the synthesis of alpha-h...
Text Solution
|
- Which of the following statements is wrong about the transesterificati...
Text Solution
|
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- Which of the following reactions would give a good yield of hydrocarbo...
Text Solution
|
- Which of the following reactions is not possible ?
Text Solution
|
- underset((A)) (PhCONH2) underset(KOBr)rarr (B) underset(MeOH)rarr (C) ...
Text Solution
|
- product (B) is :
Text Solution
|
- underset((A))(PhCOOH)underset(underset((ii)Ag(2)O //Pt//MeOH)((ii)CH(2...
Text Solution
|
- Compound (B) is :
Text Solution
|
- underset((A)) (EtCOOMe) overset("(i)" NH2 NH2 "(ii)" HNO2) underset("(...
Text Solution
|
- underset((A))("PhCOOMe") overset("(i)" NH2 OH) underset("(ii)" overset...
Text Solution
|
- Product (B) is :
Text Solution
|
- Which of the following tests would help in the distinction of HCOOH an...
Text Solution
|
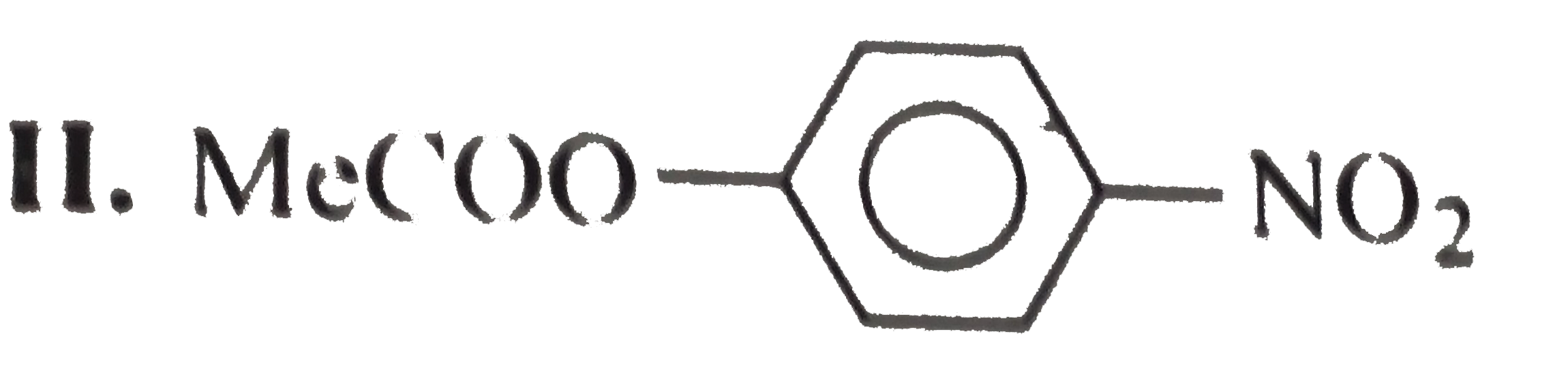
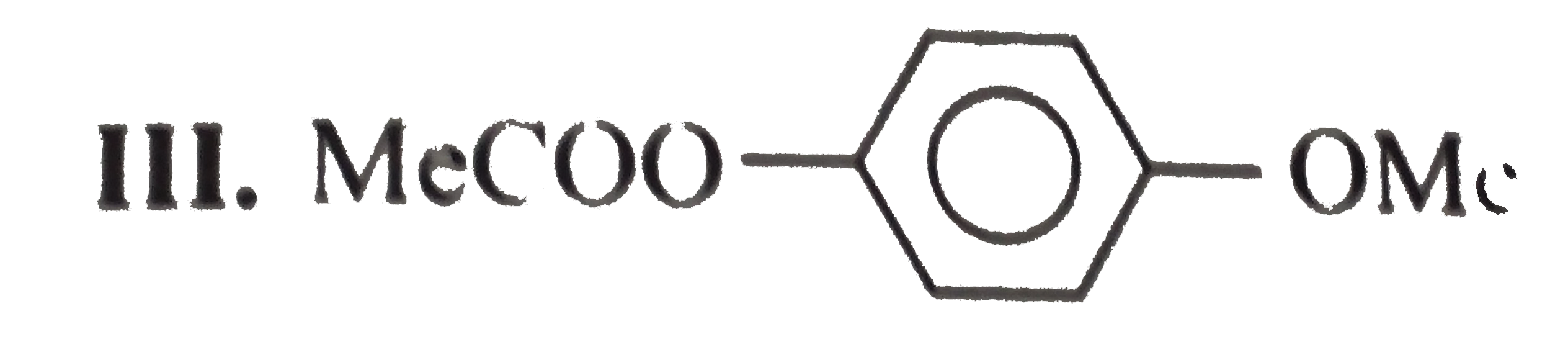
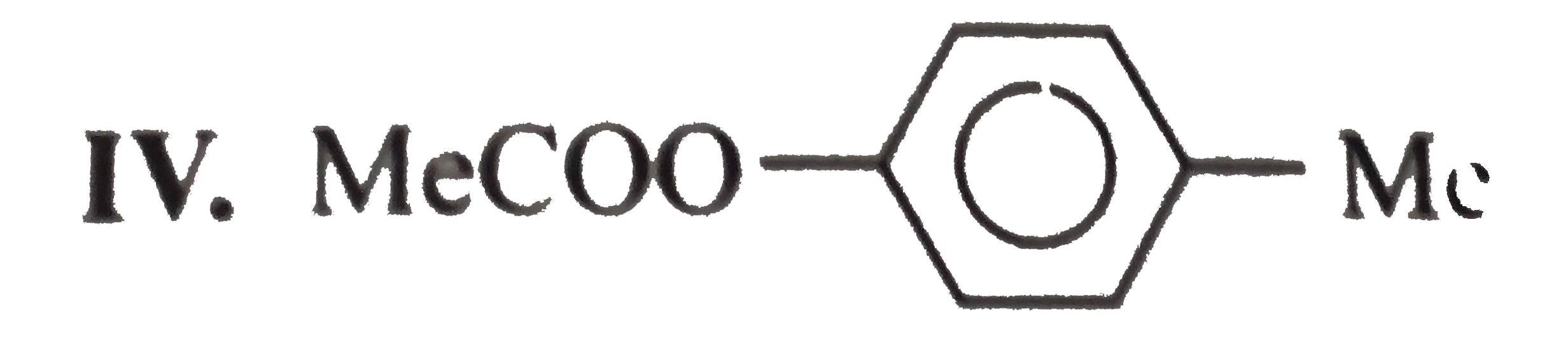 .
.