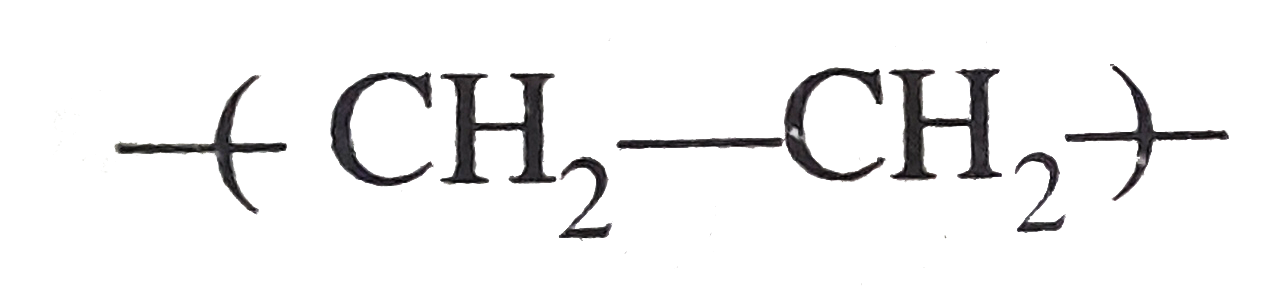A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SYNTHETIC AND NATURAL POLYMERS-Exercises Single Correct
- A polymeric sample in which30%molecules have a molecules mass 20,000,4...
Text Solution
|
- The PDI(polydispersity index) is the ratio of weight to number-average...
Text Solution
|
- The fomation of polyethylene from calcium carbide takes place as follo...
Text Solution
|
- Which one of the folowing is used to make -'non-stick'cookware?
Text Solution
|
- Whichof the following statement is not true about polymers?
Text Solution
|
- Natural rubber is a polymer of:
Text Solution
|
- Interparticle forces present in Nylon-6,6are,
Text Solution
|
- Terylene is a condensation polymer of ethylene glycol and
Text Solution
|
- Polymer used in bullet-proof glass is:
Text Solution
|
- Nylon-6 is made from:
Text Solution
|
- Which of used for the formation of nylon-6,6
Text Solution
|
- F(2)C=CF(2)is a monomer of:
Text Solution
|
- Soft drink and baby-feeding bottles are generally made of:
Text Solution
|
- Whichof the following statement is not correctly matched?
Text Solution
|
- Monomer of is:
Text Solution
|
- Which of the following is used in paints?
Text Solution
|
- Polymer fromation form monomers starts by:
Text Solution
|
- Which of the following monomer gives the polymer neoprene on polymeris...
Text Solution
|
- Acrilan is a hard material an dhas high melting point which of the fol...
Text Solution
|
- Nylon threads of made of:
Text Solution
|