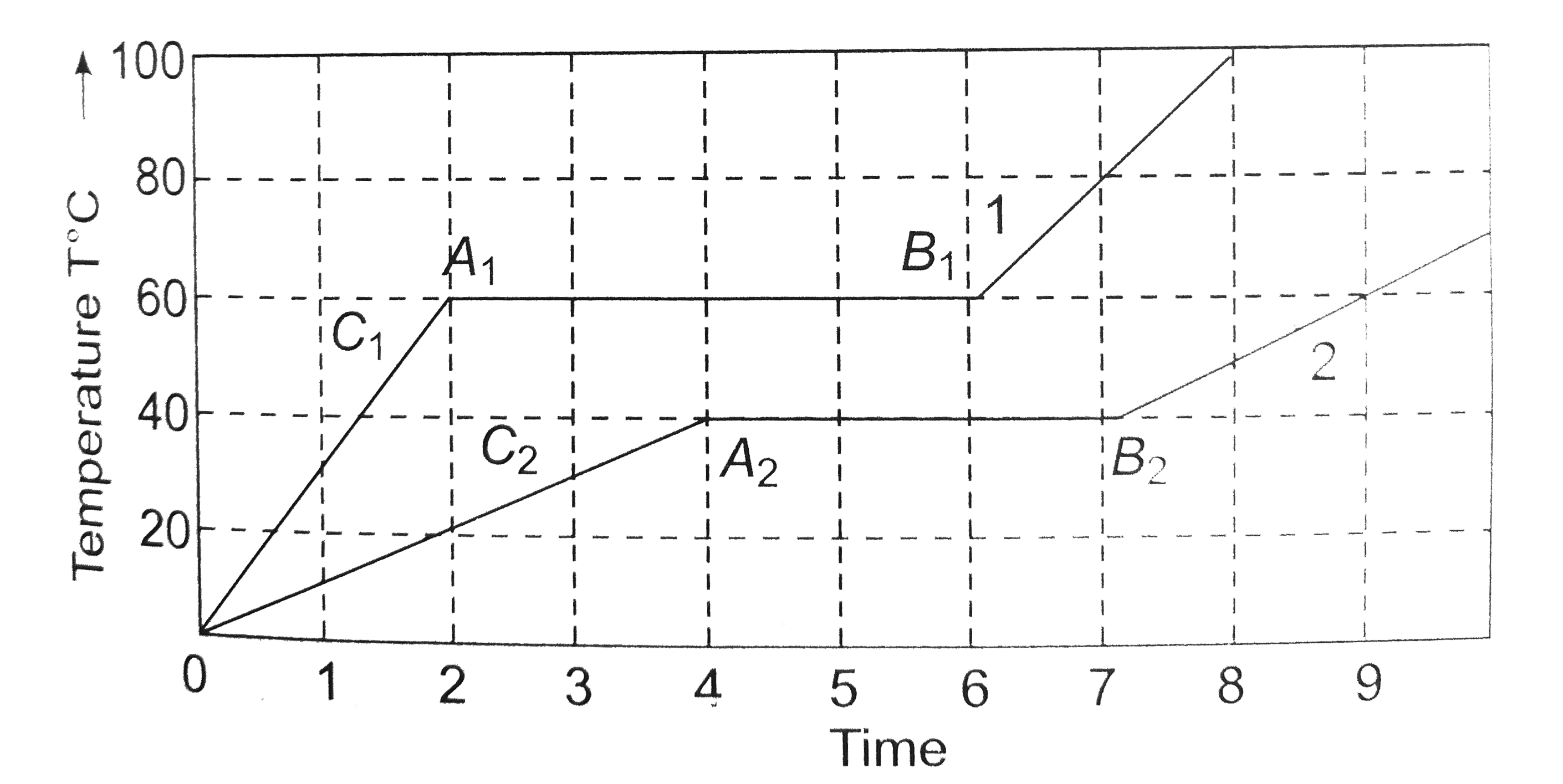
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMAL PROPERTIES OF MATTER
A2Z|Exercise Transmission Of Heat : Conduction|28 VideosTHERMAL PROPERTIES OF MATTER
A2Z|Exercise Transmission Of Heat : Radiation|28 VideosTHERMAL PROPERTIES OF MATTER
A2Z|Exercise Chapter Test|30 VideosROTATIONAL DYNAMICS
A2Z|Exercise Chapter Test|29 VideosUNIT, DIMENSION AND ERROR ANALYSIS
A2Z|Exercise Chapter Test|28 Videos
Similar Questions
Explore conceptually related problems
A2Z-THERMAL PROPERTIES OF MATTER-Calorimetry
- Equal masses of ice and water with temperature equally below and above...
Text Solution
|
- A thermal insulated vessel contains some water at 0^(@)C. The vessel i...
Text Solution
|
- Two solid bodies of equal mass m initially at T = 0^(@)C are heated at...
Text Solution
|
- A solid ball of mass 10 kg at 40^(@)C is gently placed in a liquid of ...
Text Solution
|
- An ice block at 0^(@)C is dropped from height 'h' above the ground. Wh...
Text Solution
|
- The mass, specific heat capacity and the temperature of a solid are 10...
Text Solution
|
- Four cubes of ice at -10^(@)C each one gm is taken out from the refrig...
Text Solution
|
- 20 gm ice at -10^(@)C is mixed with m gm steam at 100^(@)C. The minimu...
Text Solution
|
- The amount of heat supplied to decrease the volume of an ice water mix...
Text Solution
|
- Water of mass m(2) = 1 kg is contained in a copper calorimeter of mass...
Text Solution
|
- An ice block at 0^(@)C and of mass m is dropped from height 'h' such t...
Text Solution
|
- 4 gm of steam at 100^(@)C is added to 20 gm of water at 46^(@)C in a c...
Text Solution
|
- What is the change in potential energy (in calories) of a 10 kg mass a...
Text Solution
|
- 1 kg ice at -20^(@)C is mixed with 1 kg steam at 200^(@)C. The equilib...
Text Solution
|
- The ratio of the densities of the two bodies is 3:4 and the ratio of s...
Text Solution
|
- A 10 kW drilling machine is used to drill a bore in a small aluminium ...
Text Solution
|
- A geyser heats water flowing at the rate of 3.0 liter per minute from ...
Text Solution
|
- When 0.400 kg of water at 30^(@)C is mixed with 0.150 kg of water at 2...
Text Solution
|
- A copper calorimeter of mass 1.5 kg has 200 g of water at 25^(@)C. How...
Text Solution
|
- A copper block of mass 2.5 kg is heated in a furnace to a temperature ...
Text Solution
|