Whenever a current flows through a wire, it gets heated. According or Joule's law, heat produced in a resistance wire when a current `I` flows through it for time `t` on applying a pd (V) across its ends, is given by
`H = (VI t)/(4.2)` cal
Experimental demonstration : Verification of Joule's law
Joule's law can be very easily verified with the help of the folowing experiment. This experiment is in fact the joule's electrical method of determining `J` (Joule's mechanical equivalent of heat).
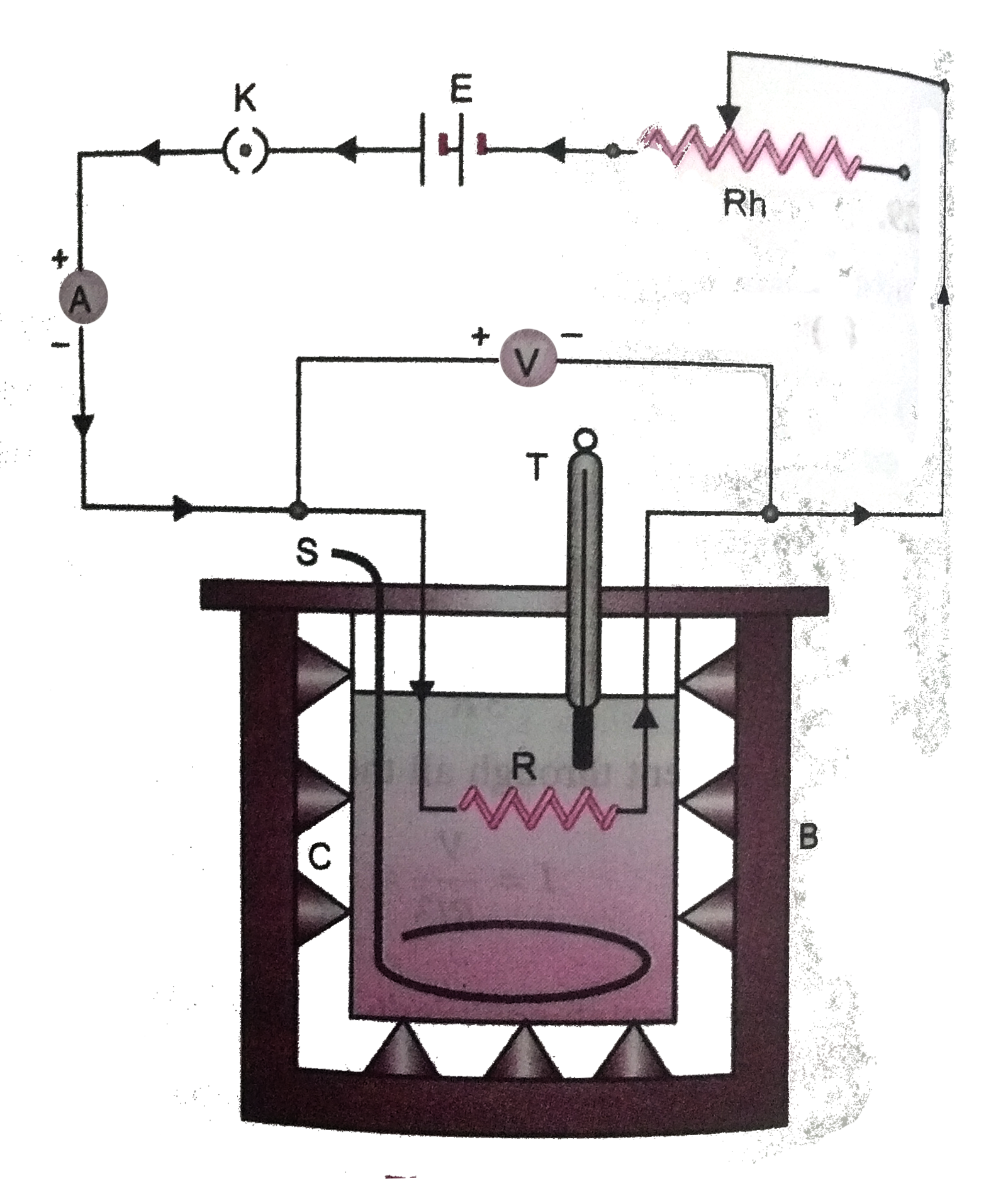
Experiment arrangement. The calorimeter C along with its stirrer S is weighed. Water is filled in the calorimeter so that the resistance wire R completely dips in it. To avoid radiation losses, the colorimeter is placed in a wooden box B. With the help of a thermomter T, the initial temperature of water is noted. The circuit in closed by plugging in the key K. With the help of the rheostat Rh, a suitable current is passed through R for a known time. The ammeter A is used to measure the current through R and a voltmeter V is used to measure pd across its ends, (Fig. 3.47).
Current is passed for so long a time as to obtain a rise of about `5^@` to `7^@ C` in the water temperature. After stopping the current flow, water is throughly stirred and its temperature is noted.
Calculations.
Let `I` = curcuit (in ampere) through the resistance R
V = pd (in volt) across the ends of the resistance
t = time (in second) for which the current flows through the wire
w = water equivalent of the calorimeter with stirrer
m = mass of water in the calorimeter
`theta_1, theta_2` = initial and final temperatures of water.
Heat gained by water `= w(theta_2 - theta_1)` cal
Heat gained by calorimeter, stirrer and water `= (w + m)(theta_2 - theta_1)` cal
Heat produced in the wire `= (VI t)/(4.2)` cal
It is found that within the limits of experimental error, `(w + m)(theta_2 - theta_1) = (VI t)/(4.2)`
Hence, Joule's Law is verified.
Applications in daily life. (i) Electric bulb, (ii) Electric oven, (iii) Electric geyser, (iv) Electric Iron.
.
 .
.