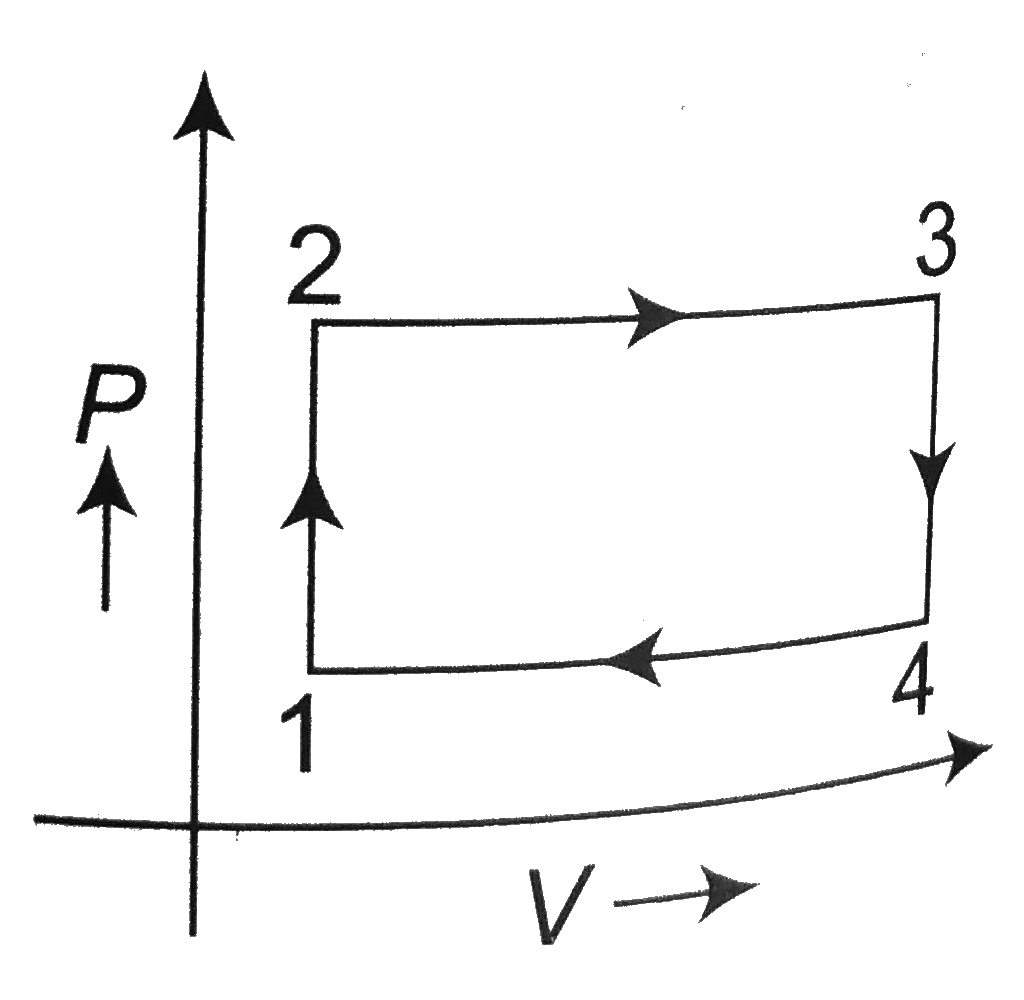
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Application Of First Law Of Thermodynamics In Different Situations|25 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Second Law Of Thermodynamics|29 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
A2Z|Exercise Ideal Gas Equation|32 VideosGRAVITATION
A2Z|Exercise Chapter Test|29 VideosMOCK TEST
A2Z|Exercise Motion With Constant Acceleration|15 Videos
Similar Questions
Explore conceptually related problems
A2Z-KINETIC THEORY OF GASES AND THERMODYNAMICS-First Law Of Thermodynamics , Internal Energy And Work Done
- An insulator container contains 4 moles of an ideal diatomic gas at te...
Text Solution
|
- Which one of the following gases possesses the largest internal energy
Text Solution
|
- In the figure given two processes A and B are shown by which a thermod...
Text Solution
|
- When a system is taken from state f along path iaf, Q = 50 J and W = 2...
Text Solution
|
- The P - V diagram of a system undergoing thermodynamic transformation ...
Text Solution
|
- The P - V diagram of 2 gm of helium gas for a certain process A rarr B...
Text Solution
|
- Volume versus temperature graph of two moles of helium gas is as shown...
Text Solution
|
- Heat is supplied to a diatomic gas at constant pressure. The ratio o...
Text Solution
|
- N moles of an ideal diatomic gas are in a cylinder at temperature T. s...
Text Solution
|
- Some of the thermodynamic parameters are state variables while some ar...
Text Solution
|
- In a process, the pressure of an ideal gas is proportional to square o...
Text Solution
|
- In a process, the pressure of an ideal gas is proportional to square o...
Text Solution
|
- A vessel contains an ideal monoatomic gas which expands at constant pr...
Text Solution
|
- Suppose 0.5 moles of an ideal gas undergoes an isothermal expansion as...
Text Solution
|
- Consider the cyclic process ABCA, shown in figure, performed on a samp...
Text Solution
|
- A quantity of heat Q is supplied to a monoatomic ideal gas which expan...
Text Solution
|
- Three moles of an ideal monoatomic gas per form a cyclic as shown in t...
Text Solution
|
- In a thermodynamic process, pressure of a fixed mass of a gas is chang...
Text Solution
|
- An ideal monoatomic gas undergoes the process AB as shown in the figur...
Text Solution
|
- One mole of a gas is subjected to two process AB and BC, one after the...
Text Solution
|