A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Archives(ASSERTION-REASIOING)|4 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Fill in the Blanks|3 VideosALKENES AND ALKADIENES
CENGAGE CHEMISTRY|Exercise Multiple Correct Answer Type|41 VideosALKANES AND CYCLOALKANES
CENGAGE CHEMISTRY|Exercise Archives|13 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALKENES AND ALKADIENES-Single Correct Answer Type
- Compound (C) is:
Text Solution
|
- Refer to Question. When the compound (B) on ozonolysis is followed by ...
Text Solution
|
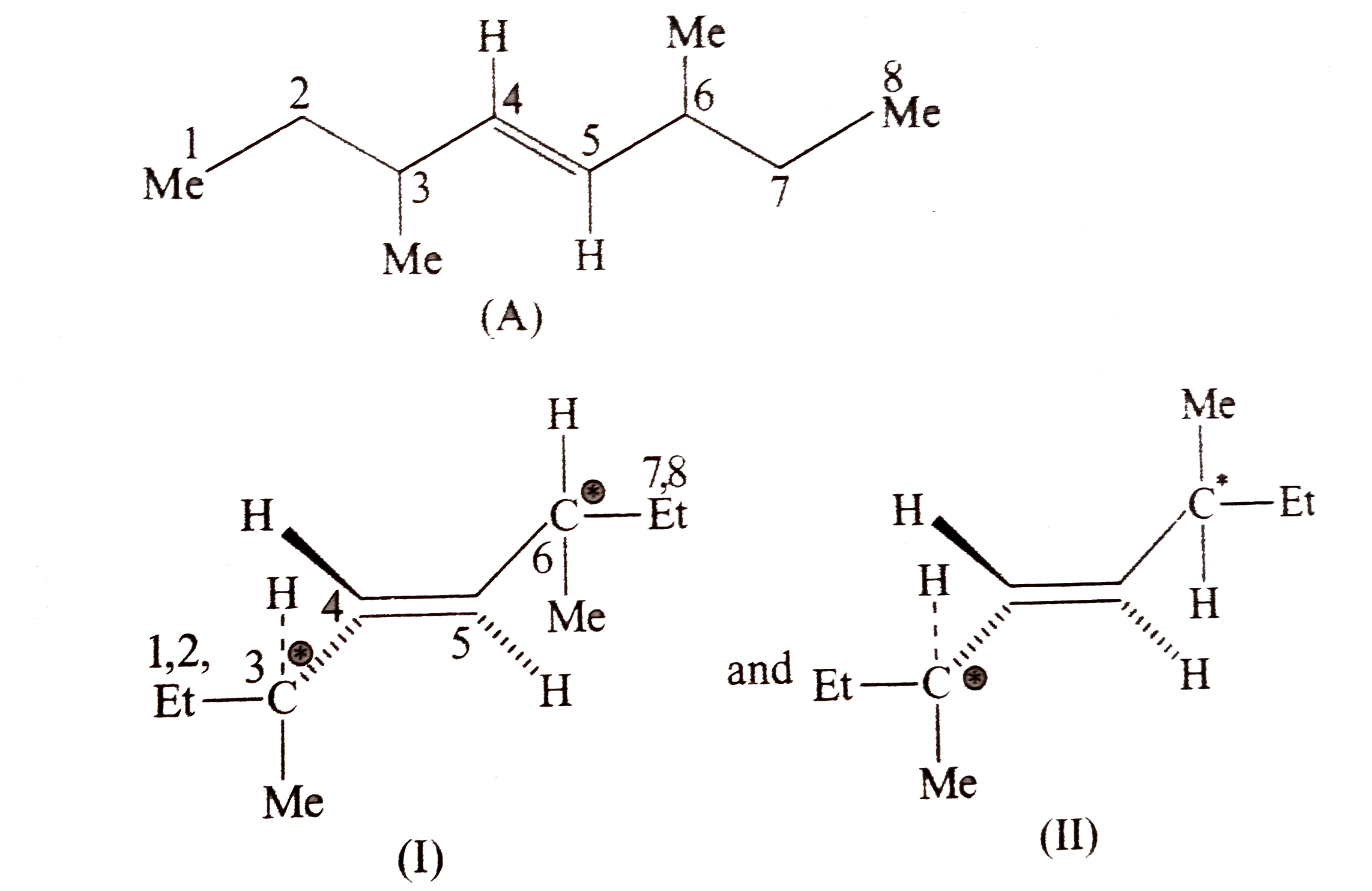
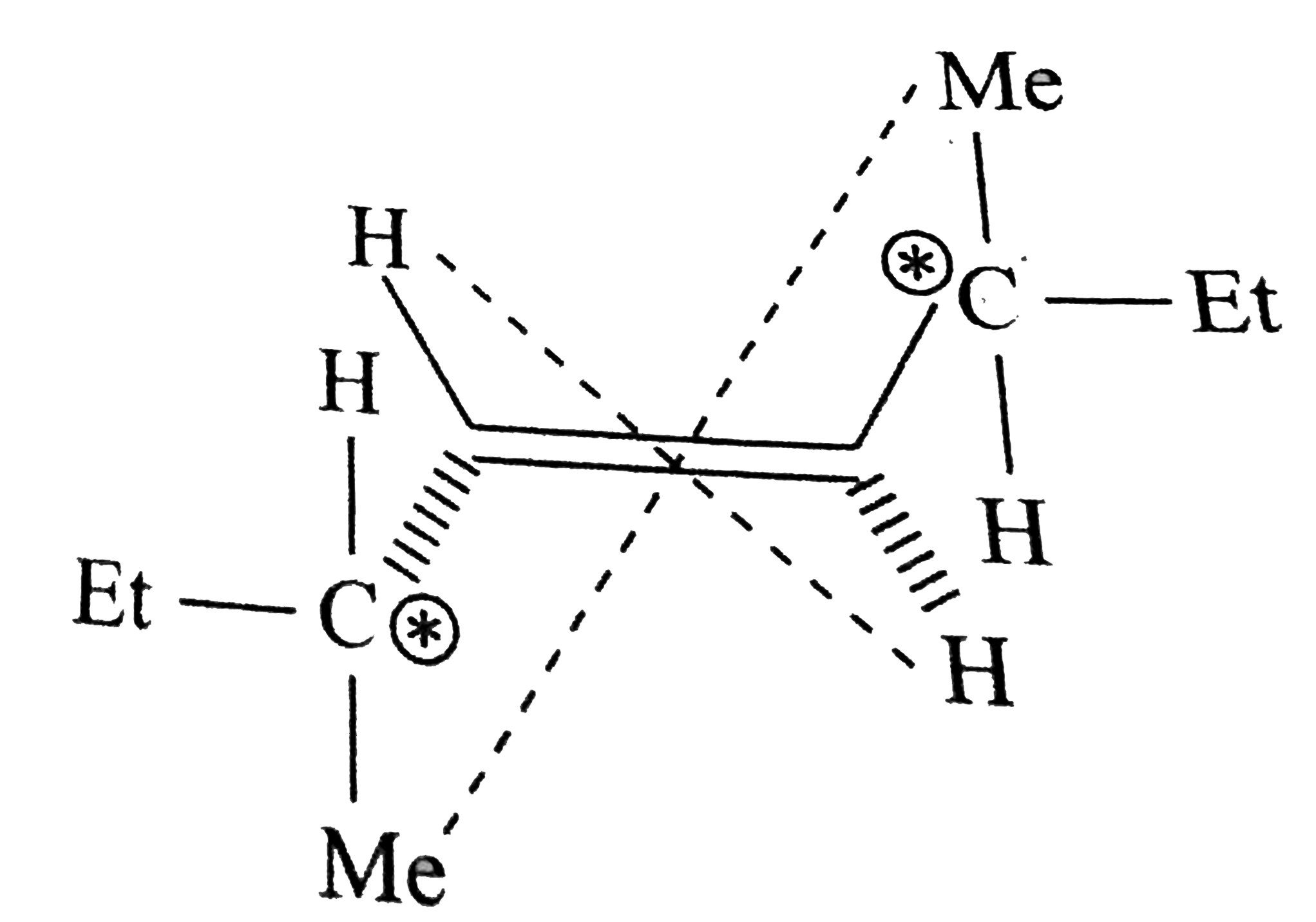
- trans -3,6-Dimethyl oct-4ene (A) exists in two diasteromer (I) and (II...
Text Solution
|
- The total number of stereoisomers for (A) is :
Text Solution
|
- If the stereochemistry about the double bond in (A) is cis-, the total...
Text Solution
|
- If the stereochemistry about the double bond in (A) is trans, two dias...
Text Solution
|
- Compounds (B) and (C) are :
Text Solution
|
- Compound (B) and (C) are :
Text Solution
|
- The three compounds (A),(B),and (C) on hydrogenation with H(2)+Pd give...
Text Solution
|
- Which of the following will have lower pK(a) value ?
Text Solution
|
- The decreasing order of acidic character is :
Text Solution
|
- Which of the following is the correct order of stability of the follow...
Text Solution
|
- Which of the following is the most stable species ?
Text Solution
|
- Which of the following is non - aromatic in nature ?
Text Solution
|
- Which of the following species is least stable ?
Text Solution
|
- Which of the following is anti- aromatic in nature ?
Text Solution
|
- Intermediate (B) and Product (C) are :
Text Solution
|
- Which statement is correct about cyclopentadienyl anion ?
Text Solution
|
- In which of the following, the DeltaH(h)^(@) is maximum ?
Text Solution
|
- Compound (C) is :
Text Solution
|