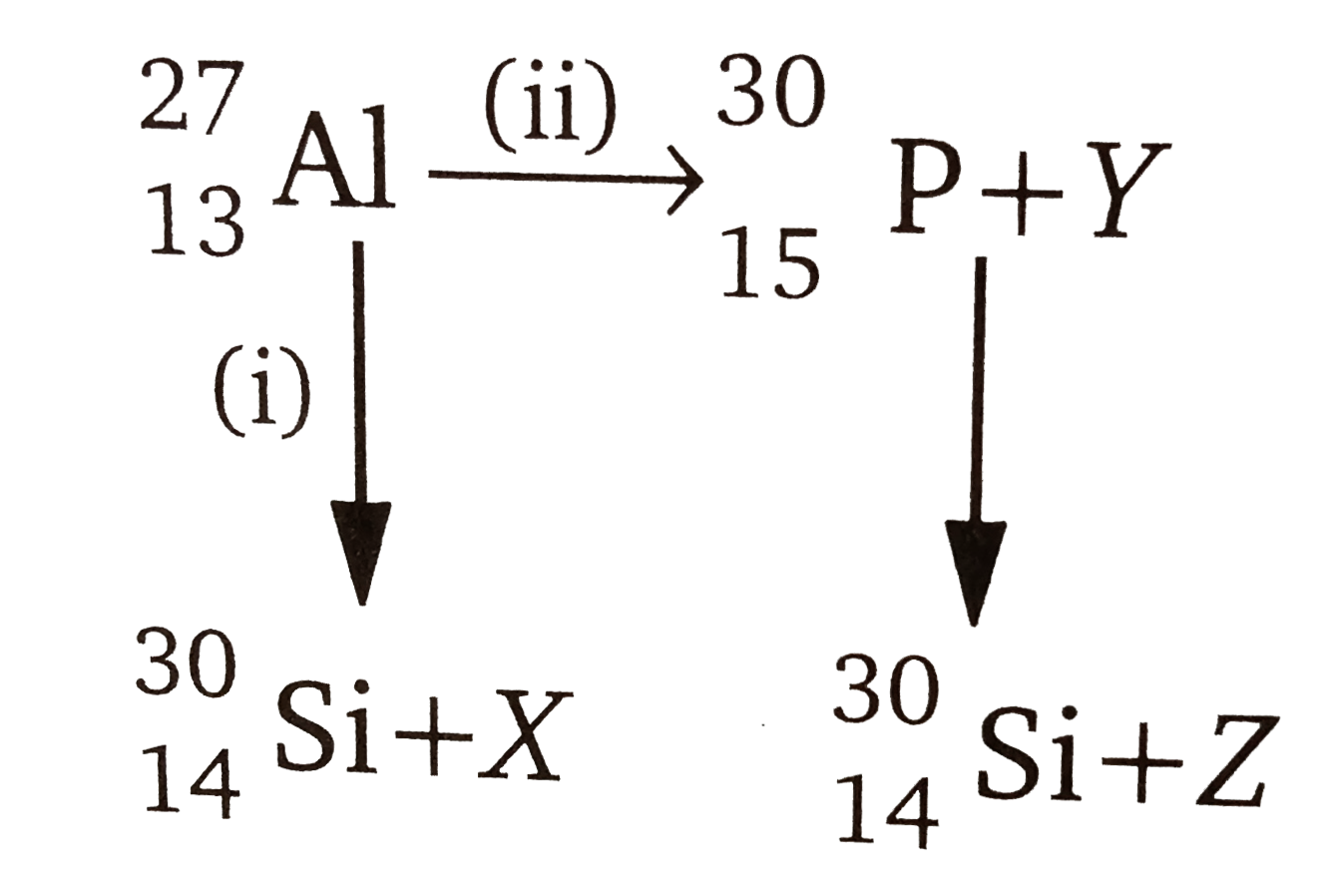A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RADIO ACTIVITY
P BAHADUR|Exercise Exercies 6|14 VideosRADIO ACTIVITY
P BAHADUR|Exercise Exercies 7|24 VideosRADIO ACTIVITY
P BAHADUR|Exercise Exercies 3B|13 VideosMOLE AND EQUIVALENT CONCEPT
P BAHADUR|Exercise Exercise 9 Advanced numerical problems|61 VideosREDOX REACTIONS
P BAHADUR|Exercise Exercise(8) Statement:Explanation type problems|19 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-RADIO ACTIVITY-Exercies 4
- The intergrated rate equation is Rt = log, C(0) - log C(t). The stra...
Text Solution
|
- beta- particle in radioactivity is emitted by:
Text Solution
|
- The half-life of a radioactive isotope is 3 hour. IF the initial mass ...
Text Solution
|
- The radioactive nuclide .(90)^(234) Th shows two successive beta- dec...
Text Solution
|
- Consider the follwing nuclear reactions: .(92)^(238) M rarr .(y)^(x)...
Text Solution
|
- The half-life of a radioisotoe is four horu. If the initial mass of th...
Text Solution
|
- Hydrogen bomb is based on the principle of
Text Solution
|
- A photon of hard gamma radiations knocks out a proton for .(12)^(24)Mg...
Text Solution
|
- In the transormation fo .(992)^(238)U to .(92)^(234)U, if one emission...
Text Solution
|
- A radioactive element gets spilled over the floor of a room. Its half ...
Text Solution
|
- Which of the follwong nuclear reactiosn will generate an isotope?
Text Solution
|
- .(13)Al^(27) is a stable isotope. .(13)Al^(29) is expected to disinteg...
Text Solution
|
- The number of neutrons accompanying the formation of .(54)Xe^(139) and...
Text Solution
|
- Decreases in atomic number is observed in:
Text Solution
|
- .^(23) Na is the more stable isotope of Na. Find out the process by wh...
Text Solution
|
- A positron is emitted from .(11)Na^(23) . The ratio of the atomic mass...
Text Solution
|
- Bombaradment of aluminium by alpha- particle leads to its artifical di...
Text Solution
|