A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
A2Z|Exercise Section B - Assertion Reasoning|13 VideosATOMIC PHYSICS
A2Z|Exercise AIPMT/NEET Questions|31 VideosATOMIC PHYSICS
A2Z|Exercise Atomic Spectrum|53 VideosALTERNATING CURRENT
A2Z|Exercise Section D - Chapter End Test|30 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC PHYSICS-Problems Based On Mixed Concepts
- alpha-particles of enegry 400 KeV are boumbardel on nucleus of .(82)Pb...
Text Solution
|
- If in Rutherford's experiment, the number of particles scattered at 90...
Text Solution
|
- An alpha-particle with a kinetic energy of 2.1eV makes a head on colli...
Text Solution
|
- An electron in hydrogen atom after absorbing an energy photon jumps fr...
Text Solution
|
- One of the lines in the emission spectrum of Li^(2+) has the same wave...
Text Solution
|
- A double charged lithium atom is equivalent to hydrogen whose atomic n...
Text Solution
|
- The ionisation potential of H-atom is 13.6 eV. When it is excited from...
Text Solution
|
- In the figure six lines of emission spectrum are shown. Which of them ...
Text Solution
|
- An orbit electron in the ground state of hydrogen has an angular momen...
Text Solution
|
- Consider atoms H, He^(+), Li^(++) in their ground states. Suppose E(1)...
Text Solution
|
- Electrons in a sample of gas containing hydrogen-like atom (Z = 3) are...
Text Solution
|
- Consider atoms H, He^(+), Li^(++) in their ground states. If L(1), L(2...
Text Solution
|
- A neutron with velocity V strikes a stationary deuterium atom, its kin...
Text Solution
|
- Imagine an atom made up of a proton and a hypotnerical particle of dou...
Text Solution
|
- If first excitation potential of a hydrogen-like atom is V electron vo...
Text Solution
|
- The energy that should be added to an electron, to reduce its de-Brogl...
Text Solution
|
- If we assume that perptraing power of any radiation/particle is invers...
Text Solution
|
- A hydrogen atom in the 4th excited state, then:
Text Solution
|
- Two hydrogen atoms are in excited state with electrons inn=2 state.Fir...
Text Solution
|
- A hydrogen like atom with atomic number Z is in an excited state of q...
Text Solution
|
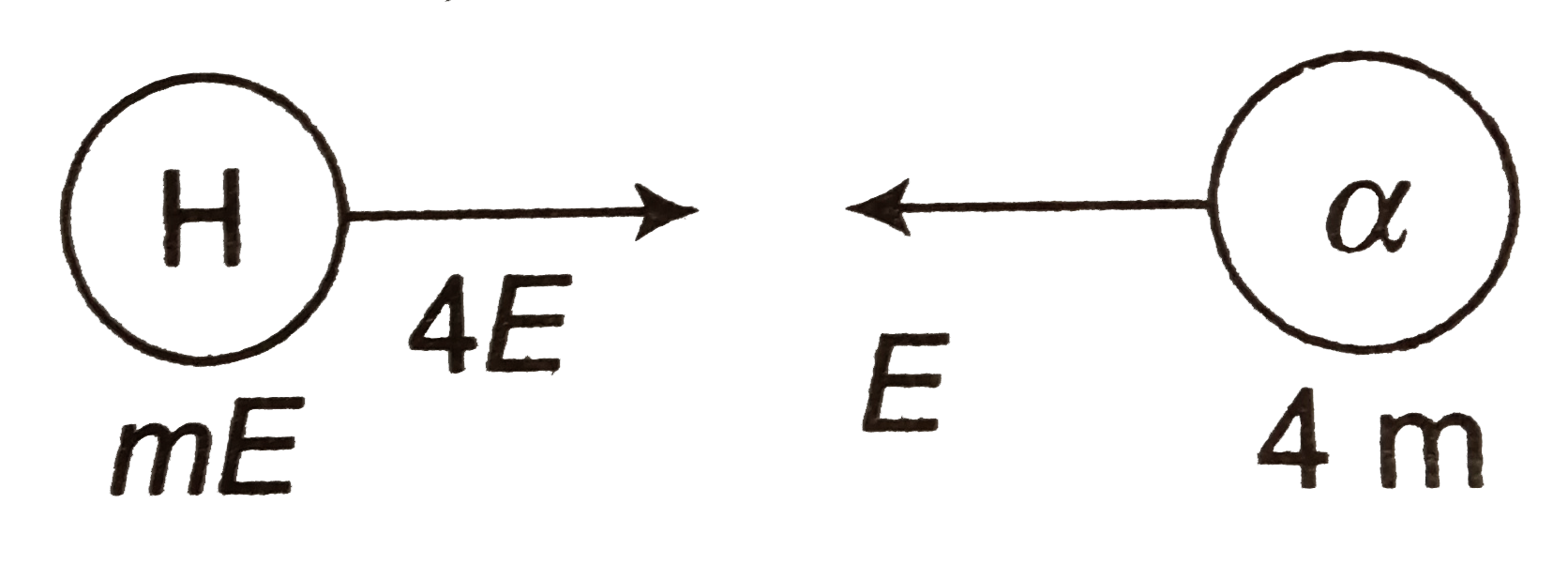 `P_(i) = sqrt(m.4.E) - sqrt(4mE) = 0`
`P_(i) = sqrt(m.4.E) - sqrt(4mE) = 0`