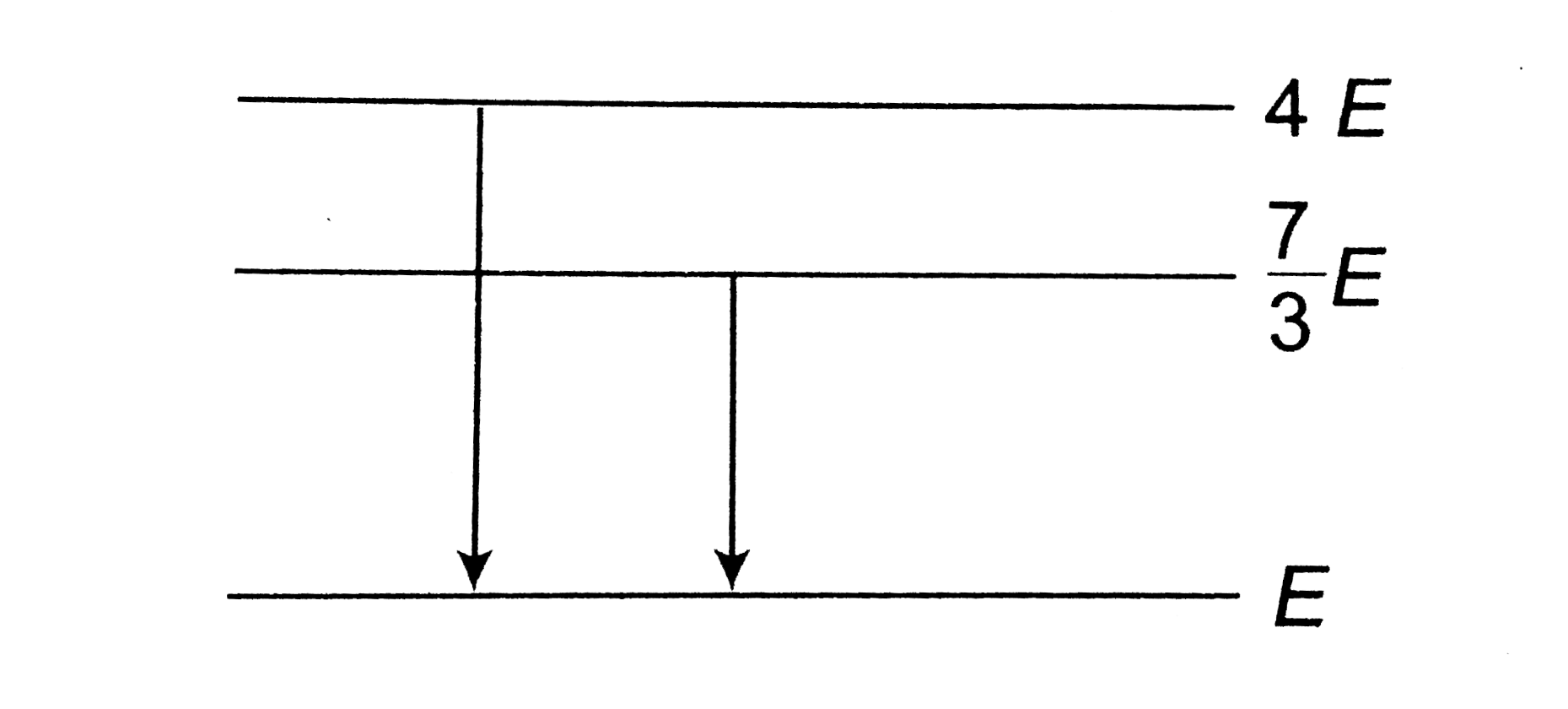
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
A2Z|Exercise Section B - Assertion Reasoning|13 VideosATOMIC PHYSICS
A2Z|Exercise AIPMT/NEET Questions|31 VideosATOMIC PHYSICS
A2Z|Exercise Atomic Spectrum|53 VideosALTERNATING CURRENT
A2Z|Exercise Section D - Chapter End Test|30 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC PHYSICS-Problems Based On Mixed Concepts
- If first excitation potential of a hydrogen-like atom is V electron vo...
Text Solution
|
- The energy that should be added to an electron, to reduce its de-Brogl...
Text Solution
|
- If we assume that perptraing power of any radiation/particle is invers...
Text Solution
|
- A hydrogen atom in the 4th excited state, then:
Text Solution
|
- Two hydrogen atoms are in excited state with electrons inn=2 state.Fir...
Text Solution
|
- A hydrogen like atom with atomic number Z is in an excited state of q...
Text Solution
|
- Consider a hydrogen-like atom whose energy in nth excited state is giv...
Text Solution
|
- The enegry level diagram for an hydrogen-like atom is shown in the fig...
Text Solution
|
- How much work must be done to pull apart the electron and the proton t...
Text Solution
|
- The ratio of ionization energy of Bohr's hydrogen atom and Bohr's hydr...
Text Solution
|
- What is the angular momentum of an electron in Bohr's hydrogen atom wh...
Text Solution
|
- In a sample of hydrogen-like atom all of which are in ground state, a ...
Text Solution
|
- A monochromatic radiation of wavelength lambda is incident on a sample...
Text Solution
|
- An electron of the kinetic energy 10 eV collides with a hydrogen atom ...
Text Solution
|
- A hydrogen atom emits a photon corresponding to an electron transition...
Text Solution
|
- The ratio between total acceleration of the electron in singly ionized...
Text Solution
|
- If the series limit of Lyman series for Hydrogen atom is equal to the ...
Text Solution
|
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- An electron beam accelerated from rest through a potential difference ...
Text Solution
|
- Imagine an atom made of a proton and a hypothetical particle of double...
Text Solution
|