A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
A2Z|Exercise AIIMS Questions|10 VideosATOMIC PHYSICS
A2Z|Exercise Assertion Reason|5 VideosATOMIC PHYSICS
A2Z|Exercise Section B - Assertion Reasoning|13 VideosALTERNATING CURRENT
A2Z|Exercise Section D - Chapter End Test|30 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-ATOMIC PHYSICS-AIPMT/NEET Questions
- Energy E of a hydrogen atom with principle quantum number n is given b...
Text Solution
|
- The energy of electron in first excited state of H-atom is -3.4 eV its...
Text Solution
|
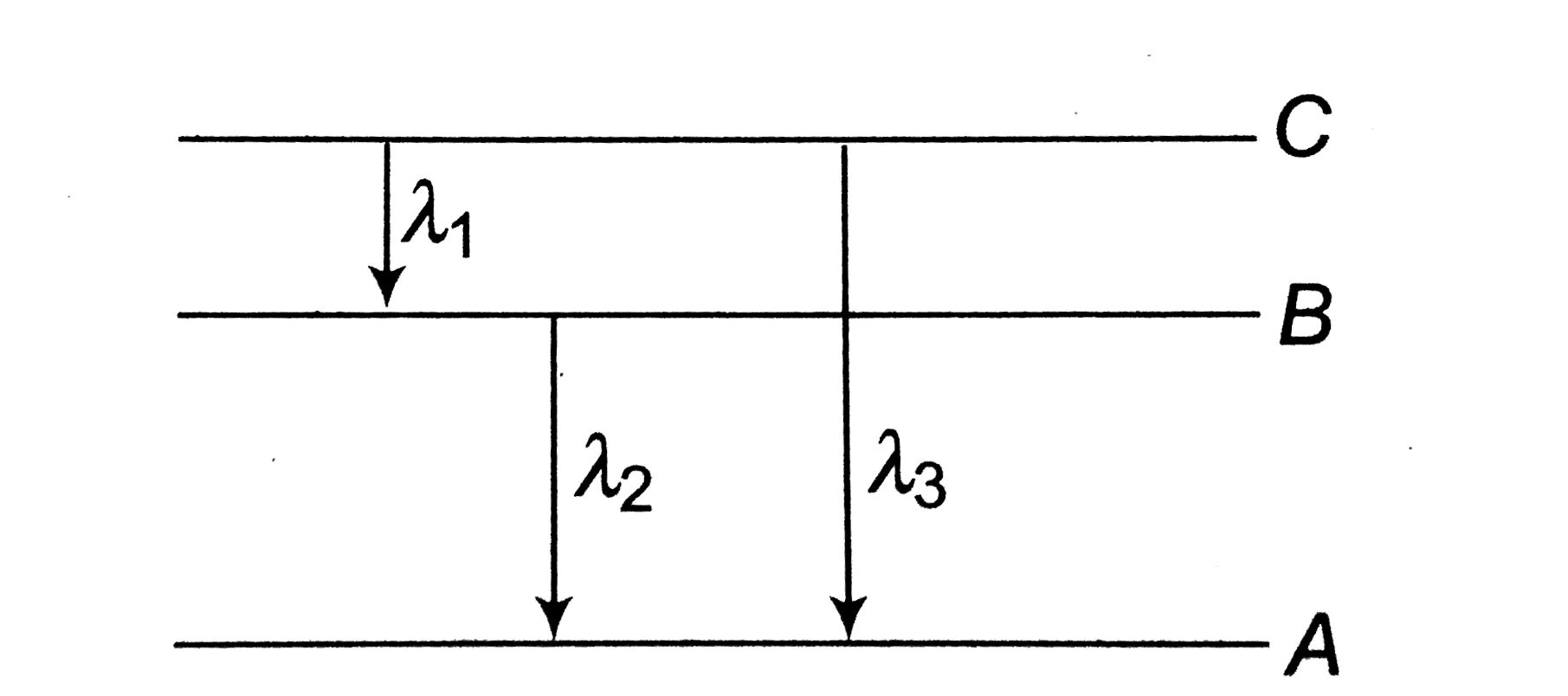
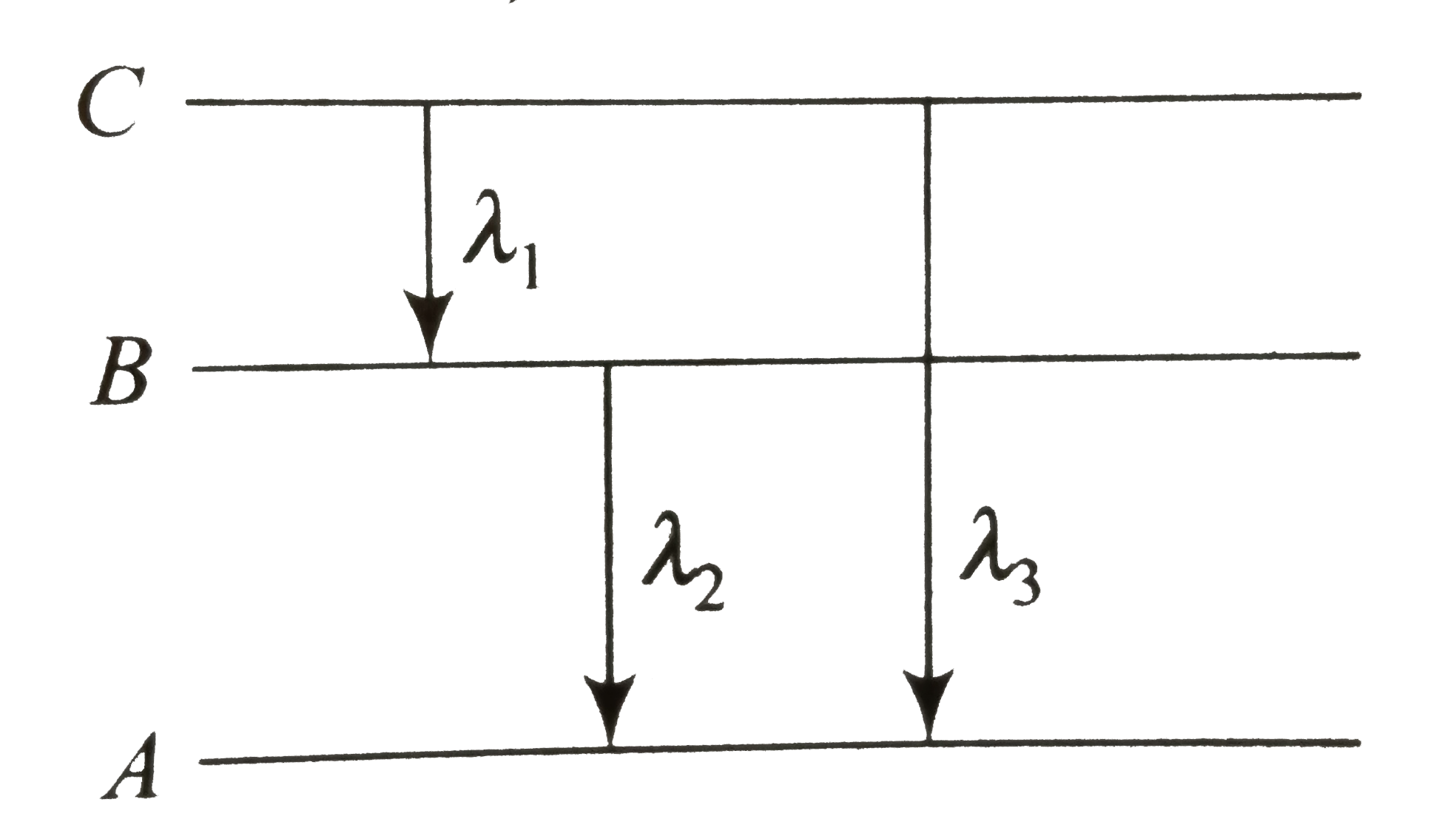
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- Ionization potential of hydrogen atom is 13.6 V. Hydrogen atoms in the...
Text Solution
|
- The total energy of eletcron in the ground state of hydrogen atom is -...
Text Solution
|
- The groud state energy of hydrogen atom is -13.6 eV. When its electron...
Text Solution
|
- In a Rutherford scattering experiment when a projectile of change Z(1)...
Text Solution
|
- The ionization enegry of the electron in the hydrogen atom in its grou...
Text Solution
|
- The energy of a hydrogen atom in the ground state is -13.6 eV. The ene...
Text Solution
|
- An alpha nucleus of energy (1)/(2)m nu^(2) bombards a heavy nucleus o...
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|
- An electron in the hydrogen atom jumps from excited state n to the gro...
Text Solution
|
- Out of the following which one is not a possible energy for a photon t...
Text Solution
|
- An electrons of a stationary hydrogen aton passes form the fifth enegr...
Text Solution
|
- Electron in hydrogen atom first jumps from third excited state to seco...
Text Solution
|
- The transition form the state n = 3 to n = 1 in a hydrogen-like atom r...
Text Solution
|
- Hydrogen atom in ground state is excited by a monochromatic radiation ...
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- Two particles of masses m(1),m(2) move with initial velocities u(1) an...
Text Solution
|
- In the spectrum of hydrogen atom, the ratio of the longest wavelength ...
Text Solution
|