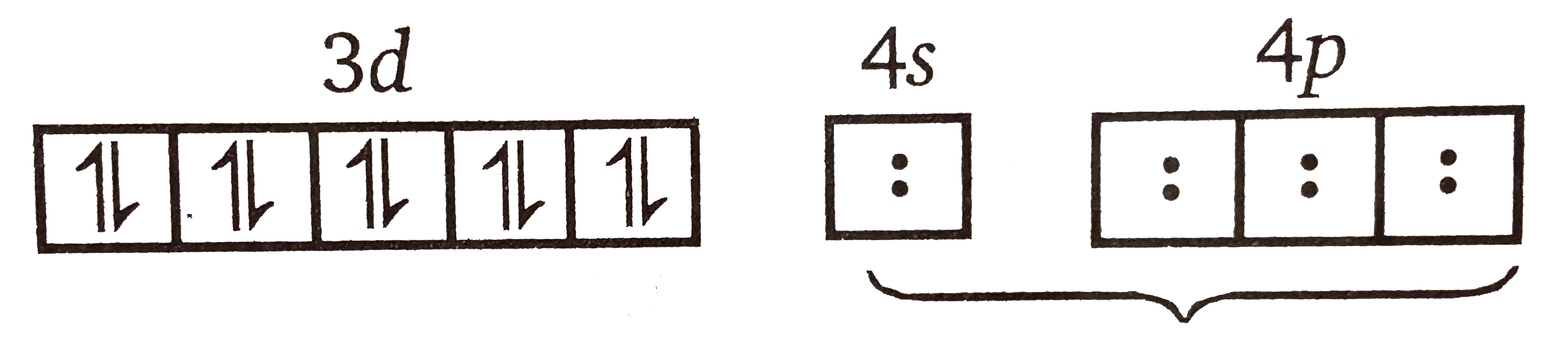
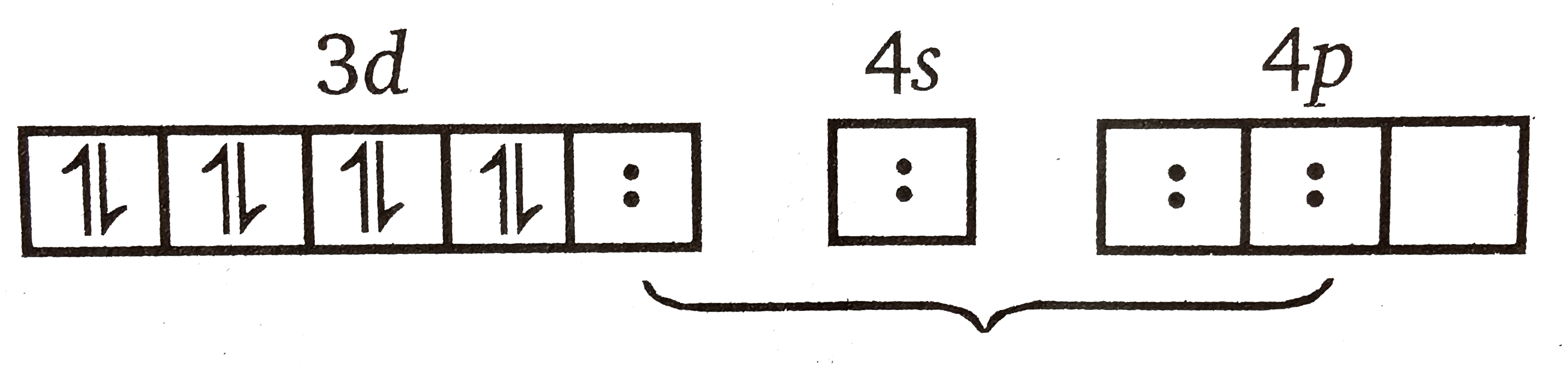
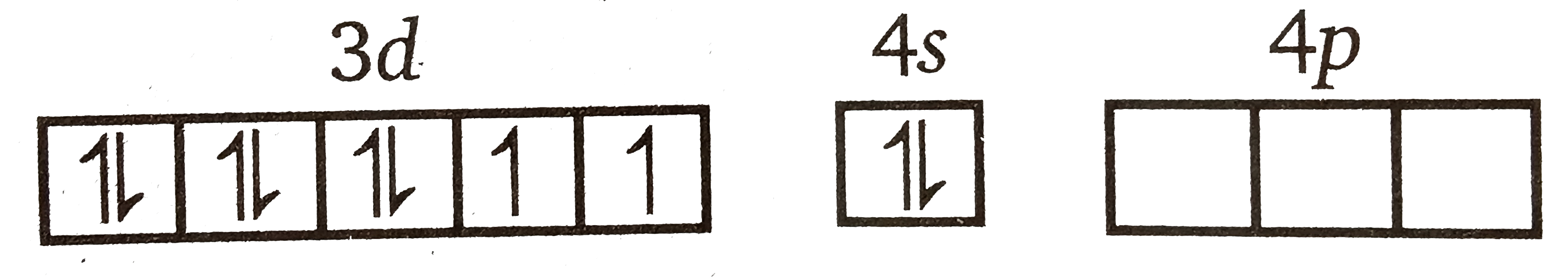A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-CHEMICAL BONDING-Exercise3
- Amongst H2O, H2S, H2 Se and H2 Te, the one with the highest biling poi...
Text Solution
|
- The correct order of hybridisation of the central atom in the oflliwng...
Text Solution
|
- The common features anong the species CN^- , CO and NO^+ are :
Text Solution
|
- Specify the co-ordination geometry around and hybridisation of N and ...
Text Solution
|
- The least stable in amongst the following is :
Text Solution
|
- Which of the following molecualr species has unpaired electrons ?
Text Solution
|
- The nodal plane is the pi -bond of ethene is located in :
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Which of the following are isoelectronics and isostructural ?
Text Solution
|
- Which of the following respresents the given mode of hybridisation sp...
Text Solution
|
- Total number of lone pair of electrons in XeOF4 is :
Text Solution
|
- Which statement is correct about O2^+?
Text Solution
|
- Which species has the maximum number of lone pair of electrons on the ...
Text Solution
|
- The species having bond order differnet from that in CO is .
Text Solution
|
- Among the following , the paramagnetic compound is :
Text Solution
|
- Hyperconjugation involves the overlapping of the following orbitals :
Text Solution
|
- Both [Ni(CO)4] and [Ni (CN)4]^(2-) are diamagnetic The hybridisation...
Text Solution
|
- The correct stability order of the following resonance structrue is : ...
Text Solution
|
- The hybridisation of atomic orbitals of nitrogen in NO2^+ , NO3^- and...
Text Solution
|
- Geometrical shapes of the complexes fromed by the reaction of Ni^(2+)...
Text Solution
|