Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-CHEMICAL BONDING-Exercise 5
- How many sigma-bonds are present in CH3 - CH3 ?
Text Solution
|
- Find the bond order of B2
Text Solution
|
- Find the bond order of Be2
Text Solution
|
- Find the bond order of N2
Text Solution
|
- Find the bond order of CO
Text Solution
|
- How many line paire of electrons are present in outer shell of Cl^- ?
Text Solution
|
- How many unpaired of electron are present in O2^-
Text Solution
|
- How many pi-bonds are present in C2 (CN)4 ?
Text Solution
|
- How many line pair of electrons are present in xeF4 ?
Text Solution
|
- A planar moelcule has ABx structrue with six paires of electrons arou...
Text Solution
|
- How many P= O bonds are in P4 O(10) ?
Text Solution
|
- How many S -O -S bonds are in S2O9 ?
Text Solution
|
- Find the formal charge of the O-atoms in [overset (..) ( :O) = N = ov...
Text Solution
|
- How many pi-bonds are in H2 S2 O6 ?
Text Solution
|
- Based on VSEPR theory , the number of 90^@ F = Br - F angles in BrR5...
Text Solution
|
- The value of n in the molecular fromula Ben Al2Si6 O(18).
Text Solution
|
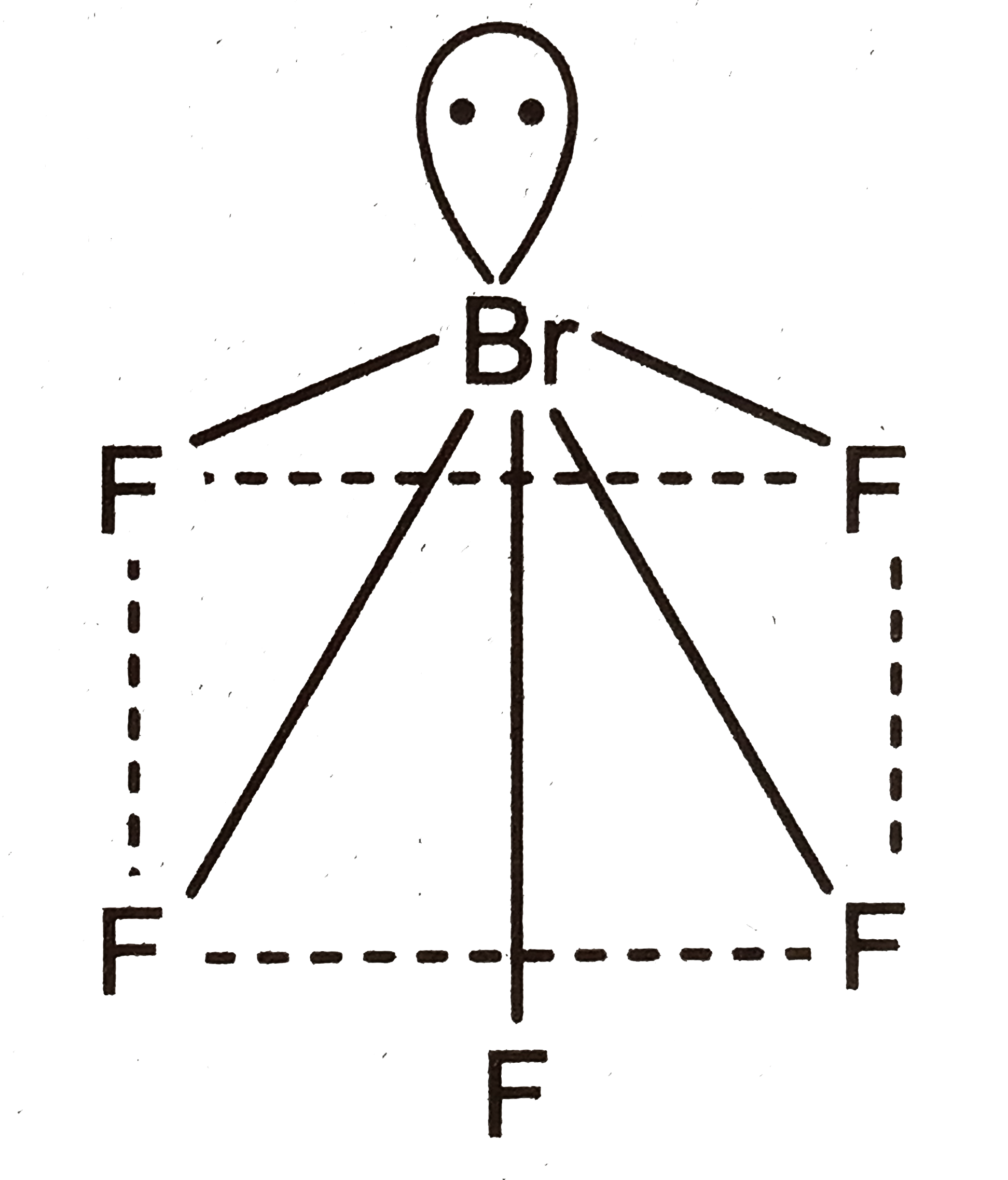 .
.