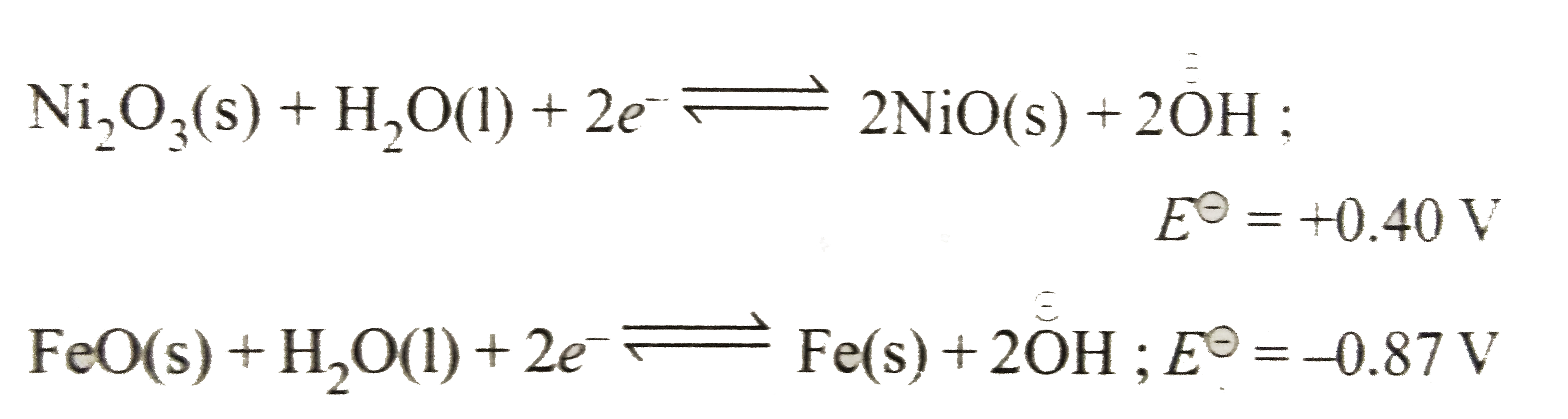Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
P BAHADUR-ELECTROCHEMISTRY-Exercise (9) ADVANCED NUMERICAL PROBLEMS
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- A current of 0.5 A is passed through acidulated water for 30 minute. C...
Text Solution
|
- A copper cell containing 5% solution of CuSO(4).5H(2)O and a silver ce...
Text Solution
|
- Some quantity of electricity being used to liberate iodine (at anode) ...
Text Solution
|
- A test for complete romovel of Cu^(2+) ions form a solution of Cu((aq....
Text Solution
|
- A Zn rod weighing 25g was kept in 100 mL of 1M CuSO(4) solution. After...
Text Solution
|
- Assume that impure copper contains only Fe, Au and Ag as impurities. A...
Text Solution
|
- 50 mL of 0.1 M CuSO(4) solution is electrolysed using Pt electrodes wi...
Text Solution
|
- An oxide of metal ("at. wt. "= 112) contain 12.5% O(2) by weight. The ...
Text Solution
|
- 19g fused SnCl(2) was electrolysed using inert electrodes. 0.119g Sn w...
Text Solution
|
- A lead storage cell is discharged which causes the H(2)SO(4) electroly...
Text Solution
|
- Two litre solution of a buffer mixture containing 1.0 M NaH(2)PO(4) an...
Text Solution
|
- A current of 40 microampere is passed through a solution of AgNO(3) fo...
Text Solution
|
- Suppose a fully charged battery containes 1.50 litre of 5.0 M H(2)SO(4...
Text Solution
|
- In an analytical determination of aresenic , a solution containing are...
Text Solution
|
- Assuming that a constant current is delivered, how many kW-h of electr...
Text Solution
|
- Find the e.m.f. of the following cell at 18^(@)C taking the degree of ...
Text Solution
|
- If it is disreed to construct the following voltaic cell to have E(cel...
Text Solution
|
- The e.m.f. of the obtained by combining Zn and Cu electrodes of Danie...
Text Solution
|
- Calcualte the potential of an indicator electrode versus the standard ...
Text Solution
|
- An electrode is prepared by dipping a silver strip into a solution sat...
Text Solution
|