Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Multiple choice questions|18 VideosTHERMODYNAMICS
PRADEEP|Exercise Problems for practice|54 VideosTHERMODYNAMICS
PRADEEP|Exercise Value based Questions|4 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-NCERT Questions
- A geyser heats water flowing at the rate of 3.0 litre per minute from ...
Text Solution
|
- What amount of heat must be supplied to 2xx10^(-2)Kg of nitrogen at ro...
Text Solution
|
- Explain why (a) Two bodies at different temperature T(1) and T(2) if...
Text Solution
|
- A cyclinder with a movable piston contains 3 mol s of hydrogen at stan...
Text Solution
|
- In changing the state of a gas adiabatically from an equilibrium state...
Text Solution
|
- Two cyclinder A and B of equal capacity are connected to eachother via...
Text Solution
|
- A steam engine delivers 5.4xx10^(8)J of work per minute and absorbs 3....
Text Solution
|
- An electric heater supplies heat to a system at a rate of 100W. If sus...
Text Solution
|
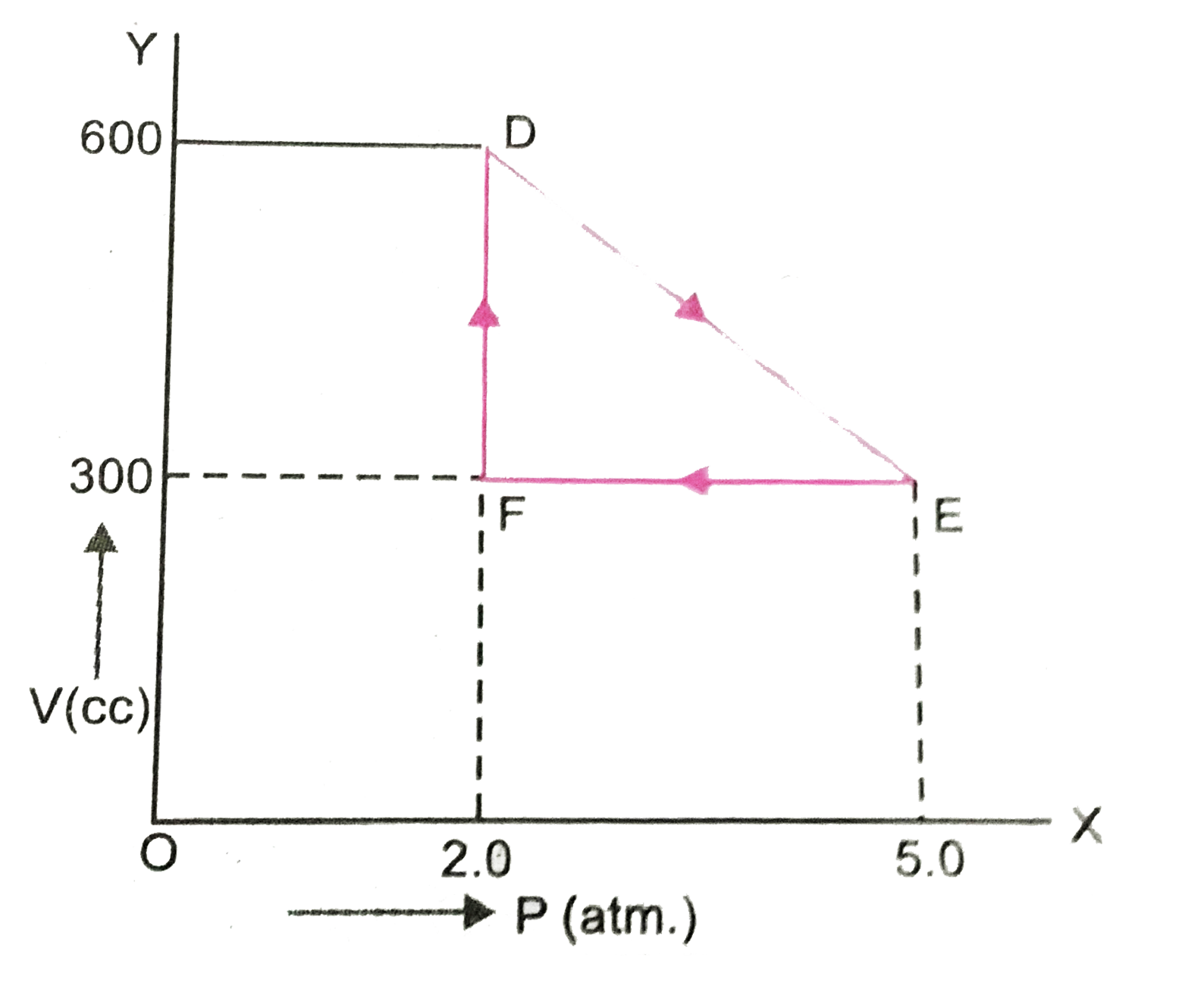
- A thermodynamic system is taken from an original state D to an interme...
Text Solution
|
- A refrigerator is to maintain eatables kept inside at 9^(@)C, if room ...
Text Solution
|