Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Multiple choice questions (NCERT)|10 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions.|96 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions|18 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Problems for practice
- Efficiency of a carnot engine is 0.4, when temp. of sink is 300K. What...
Text Solution
|
- A perfect carnot engine has source temp. 227^(@)C and sink temp. 127^(...
Text Solution
|
- An ideal engine operates by taking in steam from a boiler at 327^(@)C ...
Text Solution
|
- Two carnot engines A and B are operated in series. The first one A rec...
Text Solution
|
- A carnot engine is designed to operate between 480K and 300K. Assuming...
Text Solution
|
- A carnot engine absorbs 2000J of heat from the source of heat engine a...
Text Solution
|
- In a refrigerator, heat from inside at 270K is transferred to a room a...
Text Solution
|
- Assuming that a domestic refrigerator can be regarded as a reversivle ...
Text Solution
|
- In a cold storage, ice melts at the rate of 2 kg//h when the external ...
Text Solution
|
- A refrigerator, whose coefficient performance beta is 5, extracts heat...
Text Solution
|
- A refrigerator freezes 5 kg of water at 0^(@)C into ice at 0^(@)C in a...
Text Solution
|
- Calculate the least amount of work that must be done to freeze one gra...
Text Solution
|
- Refrigerator A works between- 10^(@)C and 27^(@)C while refrigerator B...
Text Solution
|
- In a refrigerator, heat from inside at 277 K is transferred to a room ...
Text Solution
|
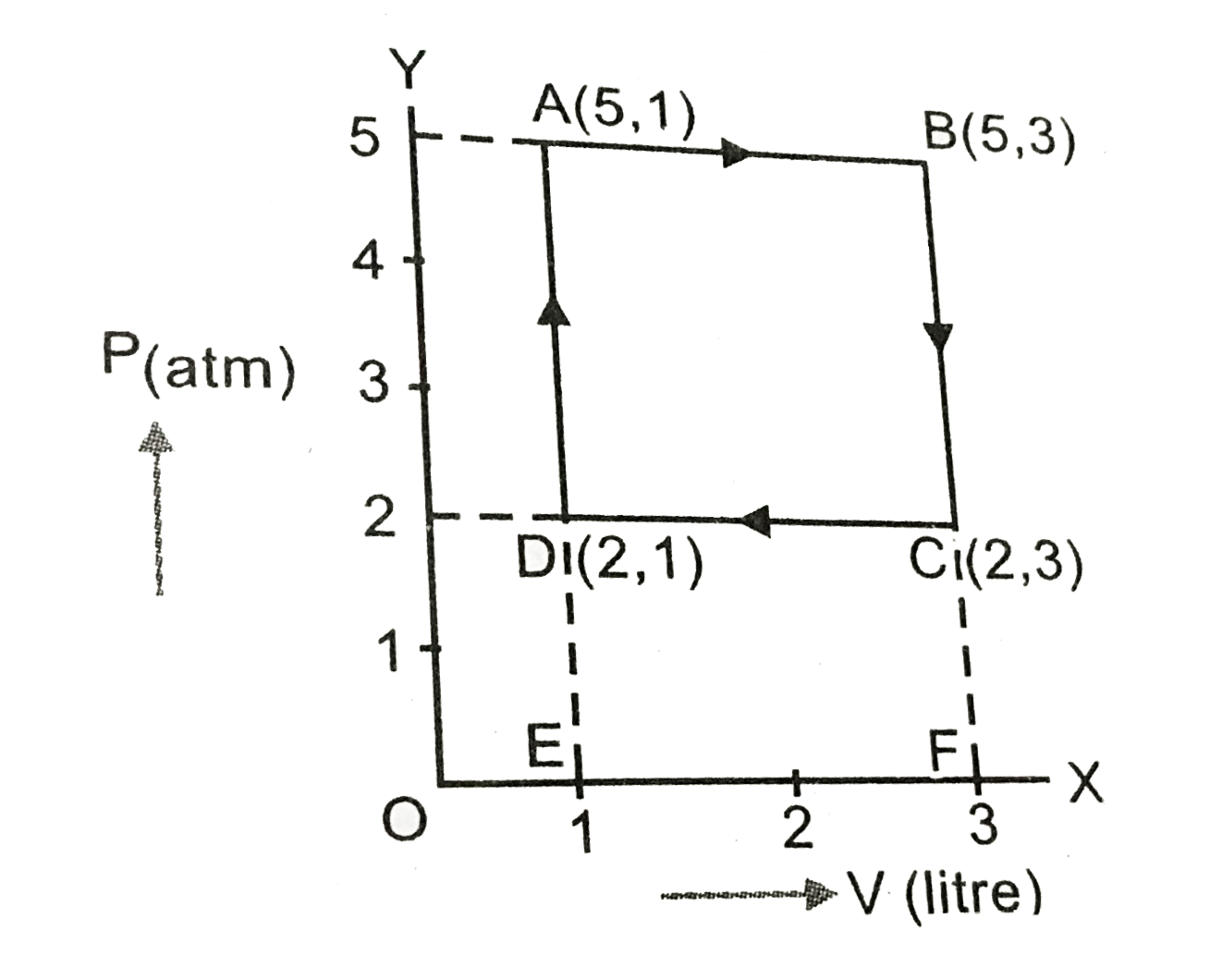
- One mole of an ideal gas undergoes a cyclic change ABCD where the (P,V...
Text Solution
|
- Calculate the heat absorbed by a system in going through the cyclic pr...
Text Solution
|
- A diatomic gas (gamma =1.4) does 200 J of work when it is expanded iso...
Text Solution
|
- Two Carnot engines are operated in succession. The first engine receiv...
Text Solution
|
- The adiabatic compression ratio in a carnot reversible cycle is 9, whe...
Text Solution
|
- Five moles of an ideal gas are taken in a Carnot engine working betwee...
Text Solution
|