Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Assertion- Reason Type Questions|19 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions.|96 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Interger Type questions
- Find the change in internal energy (in joule) of a gas when it absorbs...
Text Solution
|
- Calculate the pressure (in atm) required to compress a gas adiabatical...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic change ABCDA as shown in (...
Text Solution
|
- A carnote engine absorbs 8 KJ of energy at 400K. If sink is maintaine...
Text Solution
|
- Two carnot engine A and B operate respectively between 500 K and 400 K...
Text Solution
|
- In a refrigerator, heat from inside at 270 K is transferred to a room ...
Text Solution
|
- A refrigerator whose coefficient of performance is 12.5 extracts heat ...
Text Solution
|
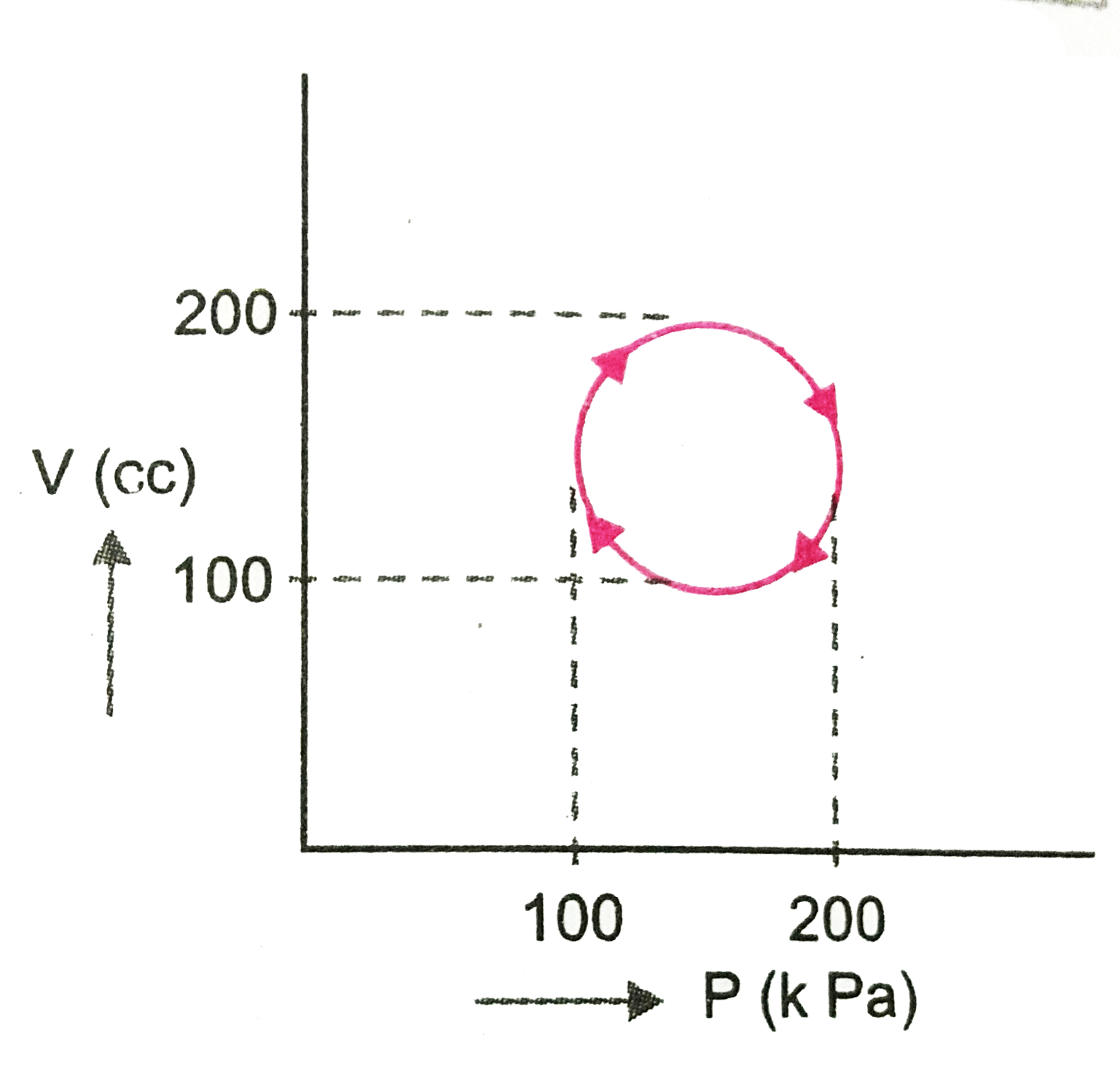
- Calculate heat (in joule) absorbed by a system going once through the ...
Text Solution
|
- During adiabatic expension of 10 mol es of a gas , internal energy dec...
Text Solution
|
- A thermodynamic system is taken from an initial state I with internal ...
Text Solution
|
- Two spherical starts A and B emit black body radiation. The radius of ...
Text Solution
|