A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Multiple choice questions-II|8 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Multiple choice questions-III|12 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Problems for practice|47 VideosGRAVIATION
PRADEEP|Exercise Assertion-Reason Type Questions|19 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BEHAVIOUR OF PERFECT GAS & KINETIC THEORY-Multiple choice questions-I
- A cubic vessel (with face horizontal + vetical ) contains an ideal gas...
Text Solution
|
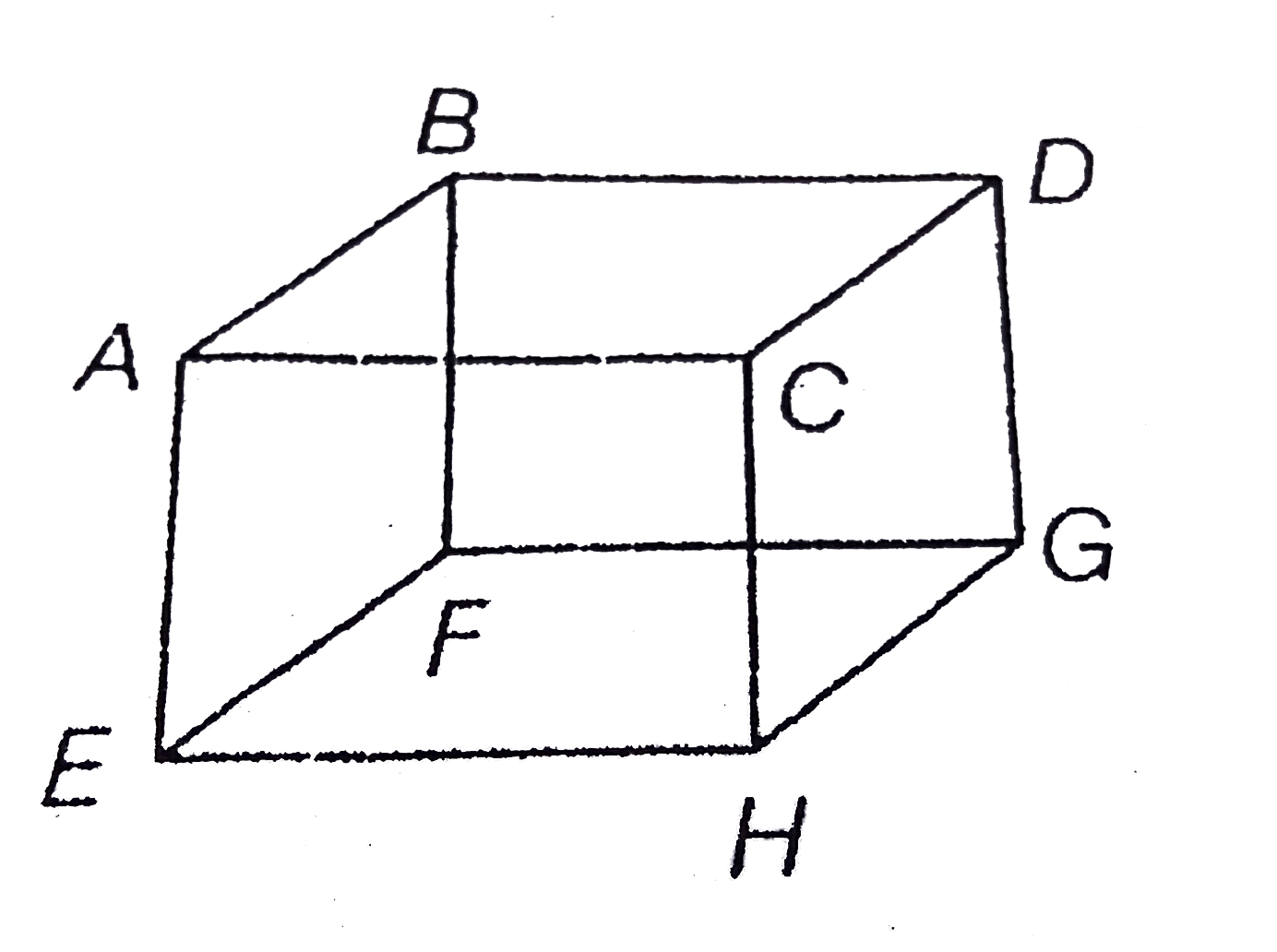
- Mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 3...
Text Solution
|
- Boyle's law is applicable for an
Text Solution
|
- A cylinder containing an ideal gas is in vertical position and has a p...
Text Solution
|
- Volume versus temperature graphs for a given mass of an ideal gas are ...
Text Solution
|
- 1 mole of H(2) gas is contained in box of volume V= 1.00 m^(3) at T = ...
Text Solution
|
- A vessel of volume V contains a mixture of 1 mole of hydrogen and 1 mo...
Text Solution
|
- An inflated rubber balloon contains one mole of an ideal gas has a pre...
Text Solution
|
- Diatomic molecules like hydrogen haven energy due to both translationa...
Text Solution
|
- In a diatomic molecule, the rotational energy at given temperature
Text Solution
|
- Which of the following diagrams, Fig. depicts ideal gas behaviour ?
Text Solution
|
- When an ideal gas is compressed adiabatically, is temperature rises th...
Text Solution
|
- A gas at 300 K has pressure 4 xx 10^(-10) N//m^(2). IF k = 1.38 xx 10^...
Text Solution
|
- 16 gram of oxygen, 14 gram of nitrogen and 11 gram of carbon dioxide a...
Text Solution
|
- A gas is found to obey the law P^(2)V = constant. The initial temperat...
Text Solution
|
- The specific heat of the mixture of two gases at constant volume is (1...
Text Solution
|
- The equation of state for 5 g of oxygen at a pressure P and temperatur...
Text Solution
|
- A certain amount of gas is sealed in a glass flask at 1 atmosphere pre...
Text Solution
|
- The temperature of an open room of volume 30 m^(3) increases from 17^(...
Text Solution
|
- When a gas filled in a closed vessel is heated through 1^(@)C, its pre...
Text Solution
|