A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Multiple choice questions-II|8 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Multiple choice questions-III|12 VideosBEHAVIOUR OF PERFECT GAS & KINETIC THEORY
PRADEEP|Exercise Problems for practice|47 VideosGRAVIATION
PRADEEP|Exercise Assertion-Reason Type Questions|19 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BEHAVIOUR OF PERFECT GAS & KINETIC THEORY-Multiple choice questions-I
- Oxygen and hydrogen gas are at same temperature and pressure. And the ...
Text Solution
|
- The average translational energy and the rms speed of molecules in a s...
Text Solution
|
- The K.E. of one mole of an ideal gas is
Text Solution
|
- At what temperature is the rms velocity of a hydrogen molecule equal t...
Text Solution
|
- The molar specific heat at constant pressure of an ideal gas is (7//2 ...
Text Solution
|
- Two rigid boxes containing different ideal gases are placed on a table...
Text Solution
|
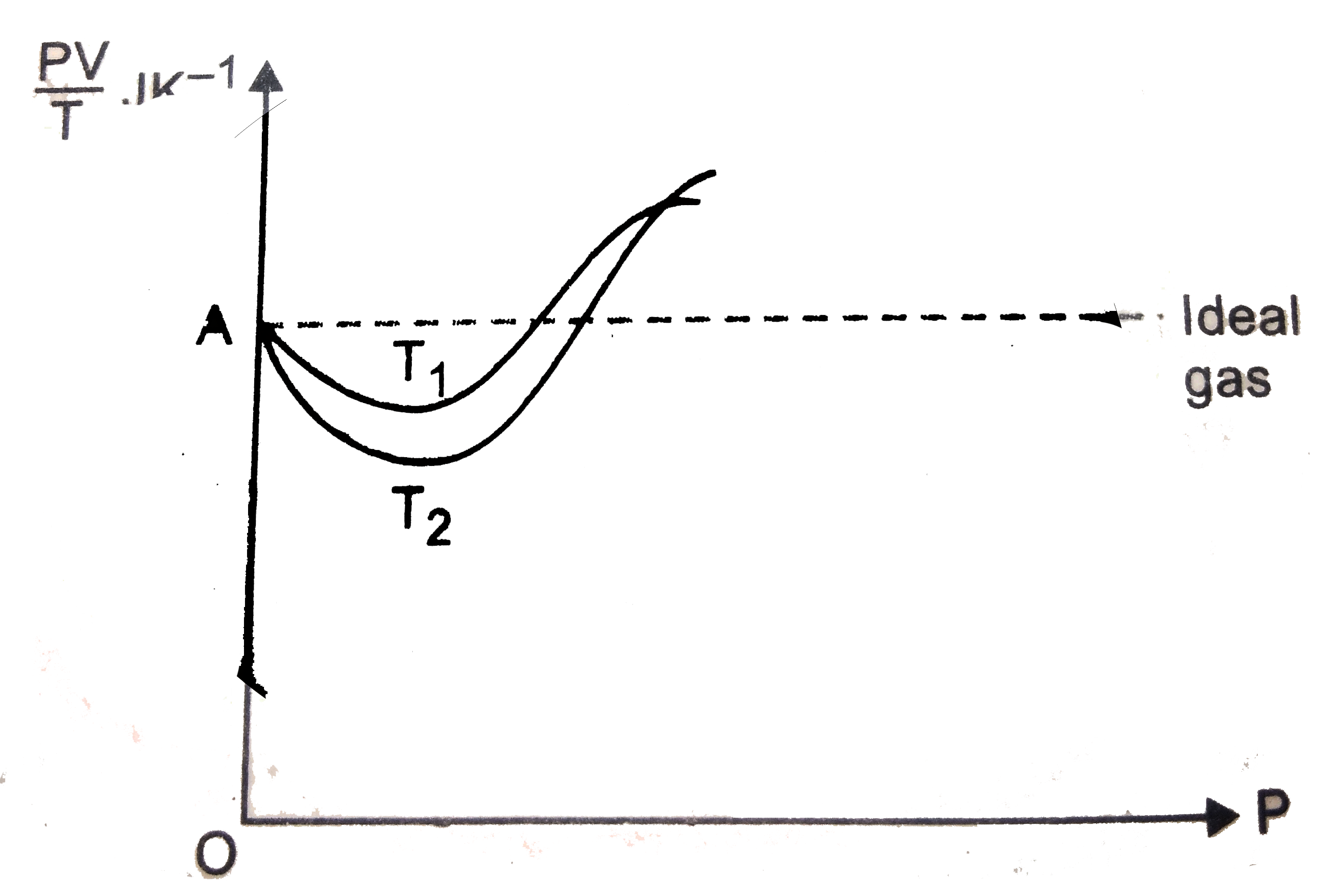
- Given is the graph between (PV)/T and P for 1 gm of oxygen gas at two ...
Text Solution
|
- The root mean square velocity of hydrogen molecules at 300 K is 1930 m...
Text Solution
|
- Two moles of oxygen are mixed with eight moles of helium. The effectiv...
Text Solution
|
- 1 mole of monoatomic and one mole of diatomic gas are mixed together. ...
Text Solution
|
- One kg of a diatomic gas is at pressure of 8xx10^4N//m^2. The density ...
Text Solution
|
- 10 moles of an ideal monoatomic gas at 10^(@)C are mixed with 20 moles...
Text Solution
|
- Find the temperature at which oxygen molecules would have the same rms...
Text Solution
|
- Mean free path of a gas molecule is
Text Solution
|
- A mixture of 2 moles of helium gas ((atomic mass)=4a.m.u) and 1 mole o...
Text Solution
|
- The molar specific heats of an ideal gas at constant pressure and volu...
Text Solution
|
- The mean free path of molecules of a gas (radius r) is inversely propo...
Text Solution
|
- The root mean square velocity of hydrogen molecule at 27^(@)C is (upsi...
Text Solution
|
- The molecules of a given mass of a gas have rms velocity of 200 m//s a...
Text Solution
|
- A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te...
Text Solution
|