A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PRADEEP-OPTICS-Exercise
- The phenomena involved in the reflected of radiowaves by ionosphere is...
Text Solution
|
- The direction of ray of light incident on a concave mirror is shown by...
Text Solution
|
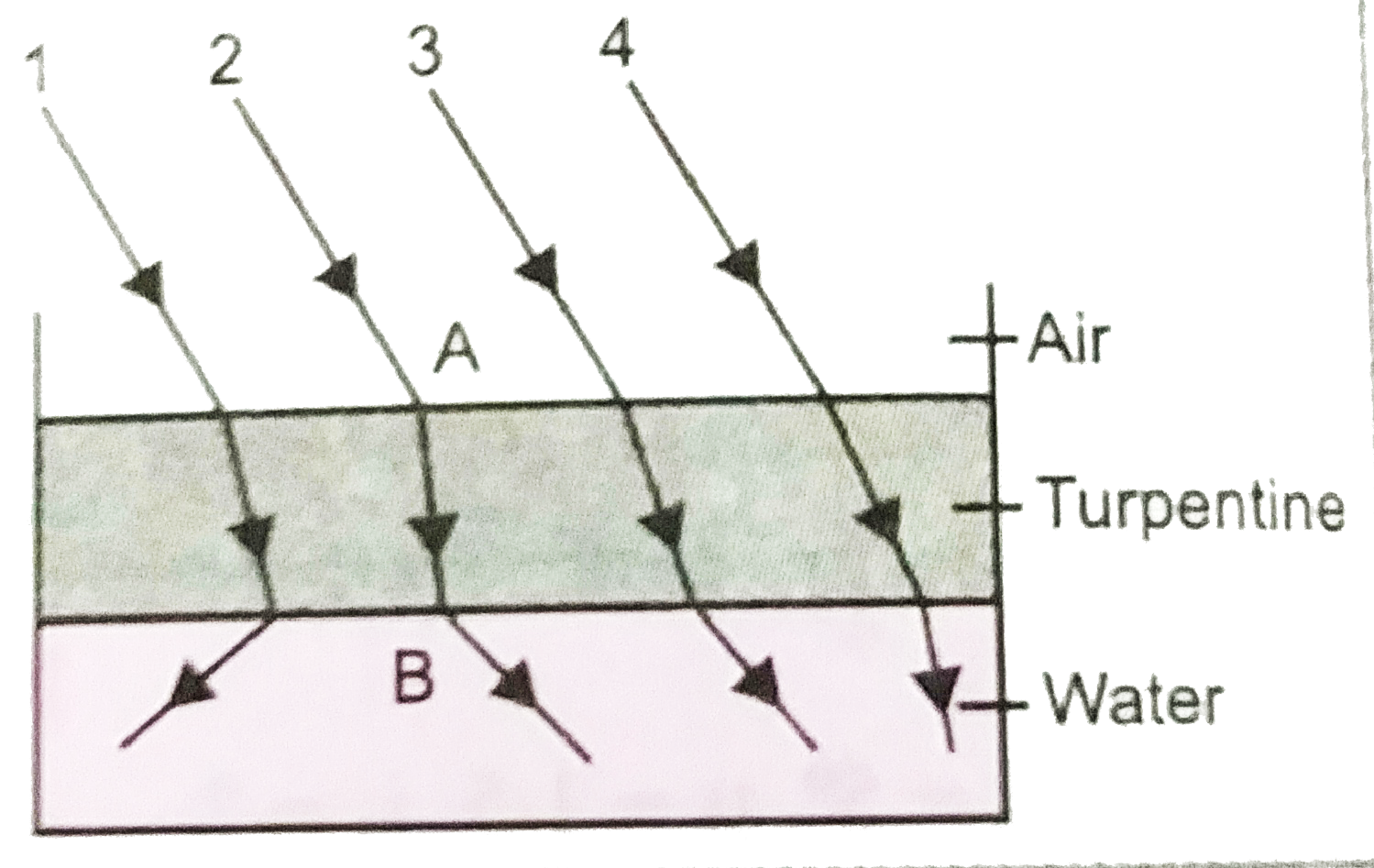
- The optical density of turpentine is higher than that of water, while ...
Text Solution
|
- A car is moving with a constant speed of 60 km h^-1 on a straight road...
Text Solution
|
- There are certain materials developed in laboratories which have a neg...
Text Solution
|
- Consider an extended object immersed in water contained in a plane thr...
Text Solution
|
- A rectangular block of glass ABCD has a refractive index 1.6. A pin is...
Text Solution
|
- Between the primary and secondary rainbows, there is a dark band known...
Text Solution
|
- A magnifying glass is used, as the object to be viewed can be brought ...
Text Solution
|
- An astronomical refractive telescope has an objective of focal length ...
Text Solution
|
- Consider a light beam incident from air to a glass slab at Brewster's ...
Text Solution
|
- Consider sunlight incident on a slit of width 10^(4) Å . The image see...
Text Solution
|
- Consider a ray of light incident from air onto a slab of glass (refrac...
Text Solution
|
- In a Young's double slit experiment, the source is white light. One of...
Text Solution
|
- Figure shows a standard two slit arrangement with slits S(1), S(2). P(...
Text Solution
|
- Two source S(1) and S(2) of intensity I(1) and I(2) are placed in fron...
Text Solution
|
- Consider sunlight incident on a pinhole of width 10^(3)Å. The image of...
Text Solution
|
- Consider the diffraction pattern for a small pinhole. As the size of t...
Text Solution
|
- For light diverging from a point source
Text Solution
|
- A source of light lies on the angle bisector of two plane mirrors incl...
Text Solution
|
 .
.