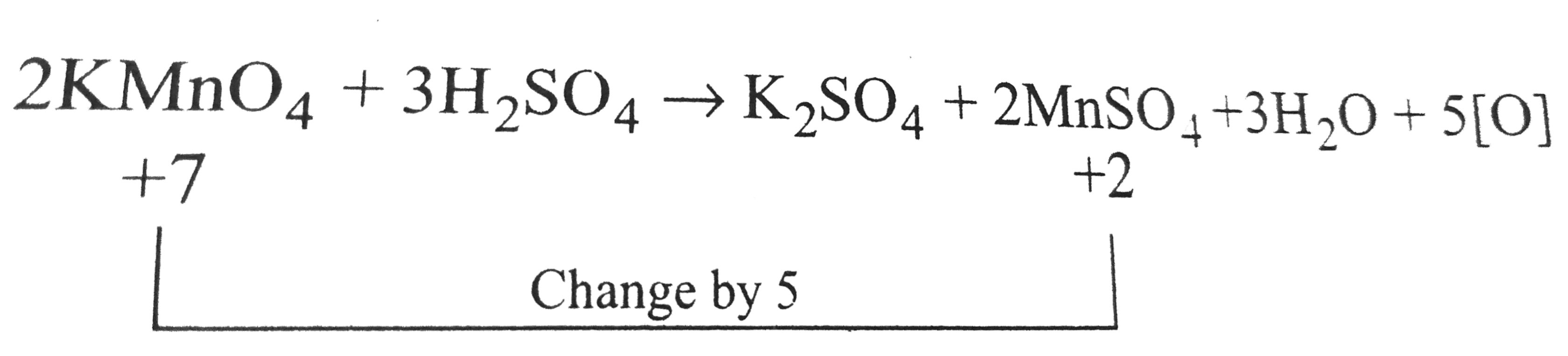A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Significant Figures, Rounding Off And Dimentional Analysis|18 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Section B - Assertion Reasoning|26 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Mole Concepts In Solution|47 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|60 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC CONCEPTS OF CHEMISTRY-Redox Titration And Stoichiometry In Various Types Of Reaction
- In the neutralization of Na(2)S(2)O(3) using K(2)Cr(2)O(7) by idometry...
Text Solution
|
- When N(2) is converted into NH(3), the equivalent weight of nitrogen w...
Text Solution
|
- Equivalent weight of KMnO(4) acting as an oxidant in acidic medium is
Text Solution
|
- In the reaction: Na(2)S(2)O(3)+4Cl(2)+5H(2)O rarr Na(2)SO(4)+H(2)SO(4)...
Text Solution
|
- In the reaction 2CuSO(4)+4KI rarr 2Cu(2)I(2)+I(2)+2K(2)SO(4) the equ...
Text Solution
|
- KMnO(4) react with oxalic acid according to the equation, 2MnO(4)^(-)+...
Text Solution
|
- 28 NO(3)^(-)+3As(2)S(3)+4H(2)O rarr 6AsO(4)^(3-)+28NO+9SO(4)^(2-)+H^(+...
Text Solution
|
- In order to prepare one litre normal solution of KMnO(4), how many gra...
Text Solution
|
- HNO(3) oxidies NH(4)^(+) ions to nitrogen and itself gets reduced to N...
Text Solution
|
- What is the concentration of nitrate ions if equal volumes of 0.1 M Ag...
Text Solution
|
- How many litres of Cl(2) at STP will be liberated by the oxidation of ...
Text Solution
|
- A solution containing Na(2)CO(3) and NaOH requires 300 ml of 0.1 N HCl...
Text Solution
|
- Which of the following cannot give iodometric titrations?
Text Solution
|
- KMnO(4) reacts with ferrous ammonium sulphate according to the equatio...
Text Solution
|
- Calculate the mass of BaCO(3) produced when excess CO(2) is bubbled th...
Text Solution
|
- The number of moles of KMnO(4) required to oxidise 1 mol of Fe(C(2)O(4...
Text Solution
|
- The ratio of amounts of H(2)S needed to precipitate all the metal ions...
Text Solution
|
- 100 ml of 0.1 N hypo decolourised iodine by the addition of x g of cry...
Text Solution
|
- How many grams of caustic potash required to completely neutralise 12....
Text Solution
|
- What should be the weight and moles of AgCl precipitate obtained on ad...
Text Solution
|