A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-STATES OF MATTER-Section D - Chapter End Test
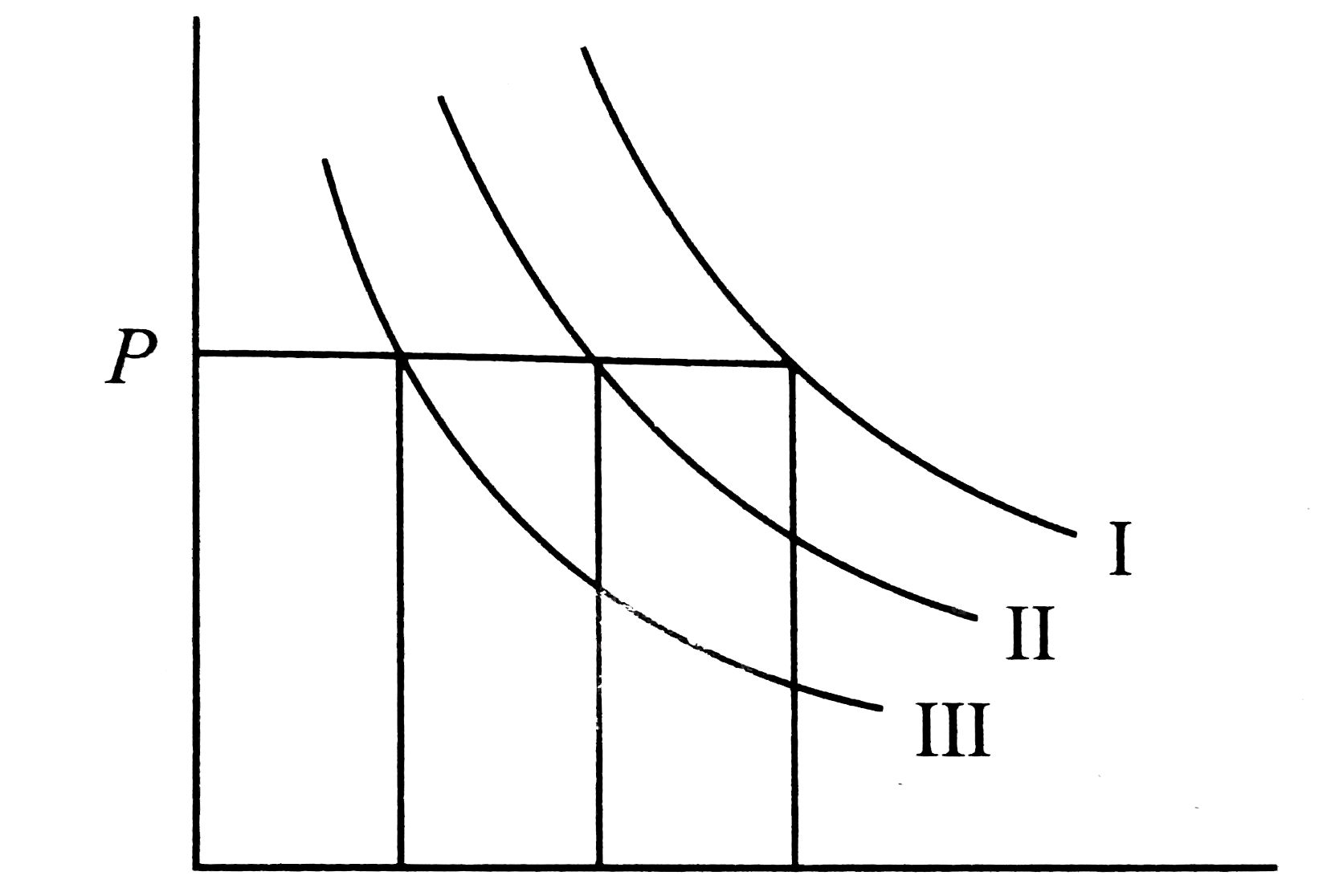
- I, II, and III are three istherms, respectively, at T(1), T(2), and T(...
Text Solution
|
- A gas can be liquefied by pressure alone when its temperature
Text Solution
|
- Boyle's law may be experssed as .
Text Solution
|
- A vessel has N(2) gas and water vapours at a total pressure of 1 atm. ...
Text Solution
|
- For two gases A and B with molecular weights M(A) and M(B), respective...
Text Solution
|
- The circulation of blood in human body supplies O(2) and releases CO(2...
Text Solution
|
- At 100^(@)C and 1 atm, if the density of the liquid water is 1.0 g cm^...
Text Solution
|
- The KE of N molecule of O(2) is x joules at -123^(@)C. Another sample...
Text Solution
|
- If for two gases of molecular weights M(A) and M(B) at temperature T(A...
Text Solution
|
- A helium atom is two times heavier than a hydrogen molecule. At 298 K,...
Text Solution
|
- Dalton's law of partial pressures is not applicable to
Text Solution
|
- The ratio between the root mean square speed of H(2) at 50 K and that ...
Text Solution
|
- Which of the following curves does not represent Boyle's law?
Text Solution
|
- The temperature of an ideal gas is increased from 140 K to 560 K. If a...
Text Solution
|
- The behaviour of a real gas is usually depicted by plotting compressib...
Text Solution
|
- XmL of H(2) gas effuses through a hole in a container is 5 second. The...
Text Solution
|
- NH(3) is liquefied more easily than N(2). Hence
Text Solution
|
- 0.2 mole sample of hydrocarbon C(x)H(y) yields after complete combusti...
Text Solution
|
- When 2 g of a gas A is introduced into an evacuated flask kept at 25^(...
Text Solution
|
- Air open vessel at 127^(@)C is heated until 1//5^(th) of air in it has...
Text Solution
|
- 3.2 g of S is heated to occupy a volume of 780 ml at 450^(@)C and 723 ...
Text Solution
|