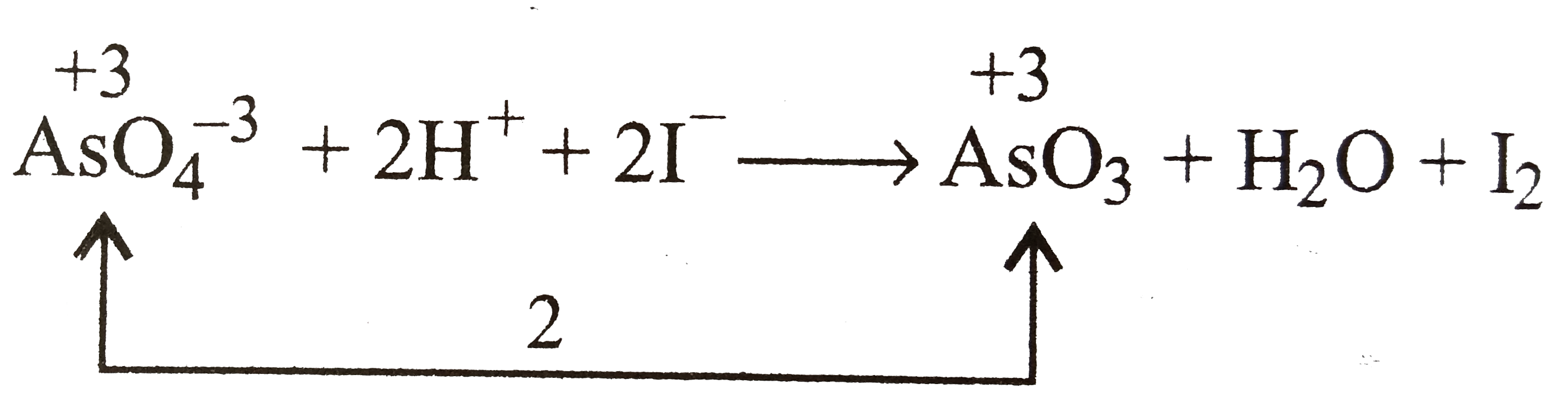A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise Type Of Redox Reaction And Equivalent Weight|42 VideosREDOX REACTIONS
A2Z|Exercise Section B - Assertion Reasoning|25 VideosREDOX REACTIONS
A2Z|Exercise Balancing Of The Equation|28 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-Stoichiometry In Redox Reactions
- 50 mL of 0.1 M solution of a salt reacted with 25 mL of 0.1 M solution...
Text Solution
|
- 4.9 g of K(2)Cr(2)O(7) is taken to prepare 0.1 L of the solutio. 10 mL...
Text Solution
|
- One gram of Na(3)AsO(4) is boiled with excess of solid KI in presence ...
Text Solution
|
- 25 mL of 0.50M H(2)O(2) solution is added to 50 mL of 0.20 M KMnO(4) i...
Text Solution
|
- 0.80 g of sample of impure potassium dichromate was dissolved in water...
Text Solution
|
- One mole of CaOCl(2) is dissolved in water andexcess of KI added. Hypo...
Text Solution
|
- An element A in a compound ABD has oxidation number A^(n-). It is oxi...
Text Solution
|
- The number of moles of KMnO(4) reduced by 1 "mol of" KI in alkaline me...
Text Solution
|
- 0.3 g of an oxalate salts was dissolved in 100 mL solution. The soluti...
Text Solution
|
- How many litres of a 0.5 N solution of an oxidising agent are reduced ...
Text Solution
|
- During the disproportionation of I(2) to iodide and iodate ions, the r...
Text Solution
|
- If 25.8 ml of 0.101 M K(2)Cr(2)O(7) is required to titrate 10.0 ml of ...
Text Solution
|
- 28 NO(3)^(-)+3As(2)S(3)+4H(2)O rarr 6AsO(4)^(3-)+28NO+9SO(4)^(2-)+H^(+...
Text Solution
|
- Moles of KHC(2)O(4) (potassium acid oxalate) required to reduce 100ml...
Text Solution
|
- The number of moles of K(2)Cr(2)O(7) that will be needed to react comp...
Text Solution
|
- 100 mL of mixture of NaOH and Na(2)SO(4) is neutralised by 10 mL of 0....
Text Solution
|
- A 0.518 g sample of limestone is dissolved in HCl and then the calcium...
Text Solution
|
- 25ml of a 0.1(M) solution of a stable cation of transition metal z rea...
Text Solution
|
- For decolourisation of 1 "mol of" KMnO(4), the moles of H(2)O(2) requi...
Text Solution
|
- In alkaline medium, ClO(2) oxidises H(2)O(2) "to" O(2) and is itself r...
Text Solution
|