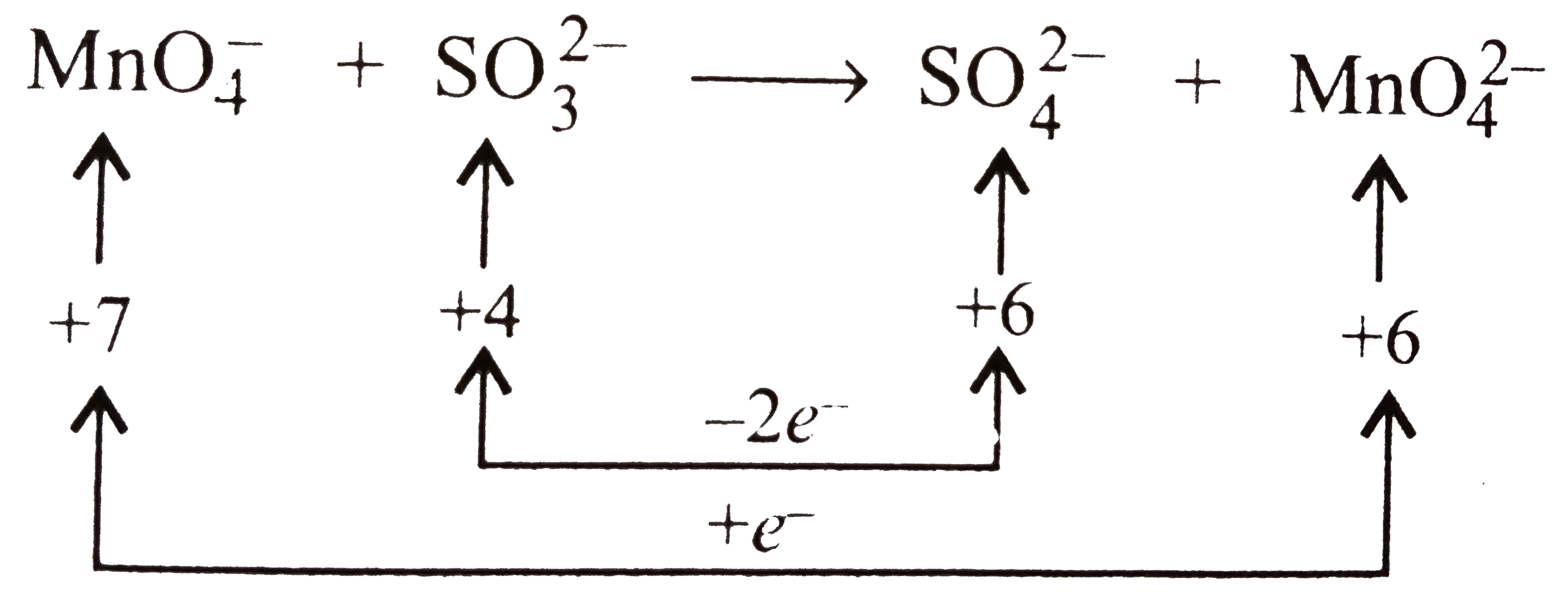A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise Type Of Redox Reaction And Equivalent Weight|42 VideosREDOX REACTIONS
A2Z|Exercise Section B - Assertion Reasoning|25 VideosREDOX REACTIONS
A2Z|Exercise Balancing Of The Equation|28 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-Stoichiometry In Redox Reactions
- In alkaline medium, ClO(2) oxidises H(2)O(2) "to" O(2) and is itself r...
Text Solution
|
- If equal volumes of 0.1 M KMnO(4) and 0.1 M K(2)Cr(2)O(7) solutions ar...
Text Solution
|
- If 10g of V(2)O(5) is dissolved in acid and is reduced to V^(2+) by zi...
Text Solution
|
- 0.45 g of acid (mol. Wt.=90) was exactly neutralized by 20 ml of 0.5(M...
Text Solution
|
- During the oxidation of arsenite to arsenate ion in alkaline medium, t...
Text Solution
|
- KMnO(4) (purple) is reduced to K(2)MnO(4) (green) by SO(3)^(2-) in bas...
Text Solution
|
- In an experiment 50ml of 0.1(M) solution of a salt is reacted with 25m...
Text Solution
|
- How many litres of Cl(2) at STP will be liberated by the oxidation of ...
Text Solution
|
- When the ion Cr(2)O(7)^(2-) acts as an oxidant in acidic aqueous solut...
Text Solution
|
- MnO(4)^(2-) (1 mole) in neutral aqueous medium is disproportionate to
Text Solution
|
- What volume of 3 molar HNO(3) is needed to oxidise 8 g of Fe^(3+), HNO...
Text Solution
|
- The number of moles of KMnO(4) that will be needed to react with one m...
Text Solution
|
- How many litres of Cl(2) at STP will be liberated by the oxidation of ...
Text Solution
|
- HNO(3) oxidies NH(4)^(+) ions to nitrogen and itself gets reduced to N...
Text Solution
|
- What volume (in ml) at STP of SO(2) gas is oxidized by 100 ml of 0.1 (...
Text Solution
|
- What mass of N(2)H(4) can be oxidised to N(2) by 24 g of K(2)CrO(4) wh...
Text Solution
|
- The number of mole of oxalate ions oxidised by one mole of MnO(4)^(-) ...
Text Solution
|
- Starch iodide paper is used to test for the presence of
Text Solution
|
- What weight of HNO(3) is needed to convert 5g of iodine into iodic aci...
Text Solution
|
- The molar ration of Fe^(++) to Fe^(+++) in a mixture of FeSO(4) and Fe...
Text Solution
|