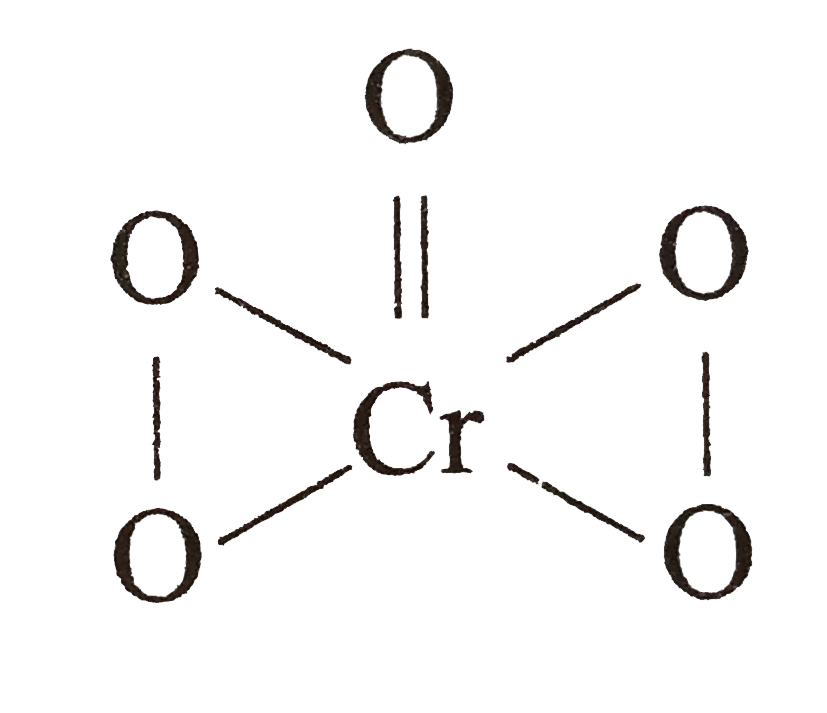
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise AIIMS Questions|20 VideosREDOX REACTIONS
A2Z|Exercise Assertion - Reasoning Questions|5 VideosREDOX REACTIONS
A2Z|Exercise Section B - Assertion Reasoning|25 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-AIPMT/ NEET Questions
- Zn gives H(2) gas with H(2)SO(4) and HCl but not with HNO(3) because
Text Solution
|
- The oxidation states of sulphur in the anions SO(3)^(2-), S(2)O(4)^(2-...
Text Solution
|
- Which is the best description of the behaviour of bromine in the react...
Text Solution
|
- Oxidation numbers of P in PO4^(3-), of S in SO4^(2-), and that of Cr...
Text Solution
|
- Oxidation no. of P in H(4)P(2)O(5), H(4)P(2)O(6), and H(4)P(2)O(7) are...
Text Solution
|
- The most common and stable oxidation state of a lanthanide is
Text Solution
|
- Standard reduction potentails of the half reactions are given below: ...
Text Solution
|
- In which of the following compounds, nitrogen exhibits the highest oxi...
Text Solution
|
- When Cl(2) gas reacts with hot and concentrated sodium hydroxide solut...
Text Solution
|
- A mixture of potassium chlorate, oxalic acid and sulphuric acid is hea...
Text Solution
|
- The pair of compounds that can exist together is:
Text Solution
|
- Role of hydrogen peroxide iin the following reaction is respectively. ...
Text Solution
|
- In acidic medium, H(2)O(2) changes Cr(2)O(7)^(2-) to CrO(5) which has ...
Text Solution
|
- The reaction of aqueus KMnO(4) with H(2)O(2) in acidic conditions give...
Text Solution
|
- For the redox reaction MnO(4)^(ө)+C(2)O(4)^(2-)+H^(o+)rarrMn^(2+)+CO...
Text Solution
|
- Which ordering of compound is according to the decreasing order of the...
Text Solution
|