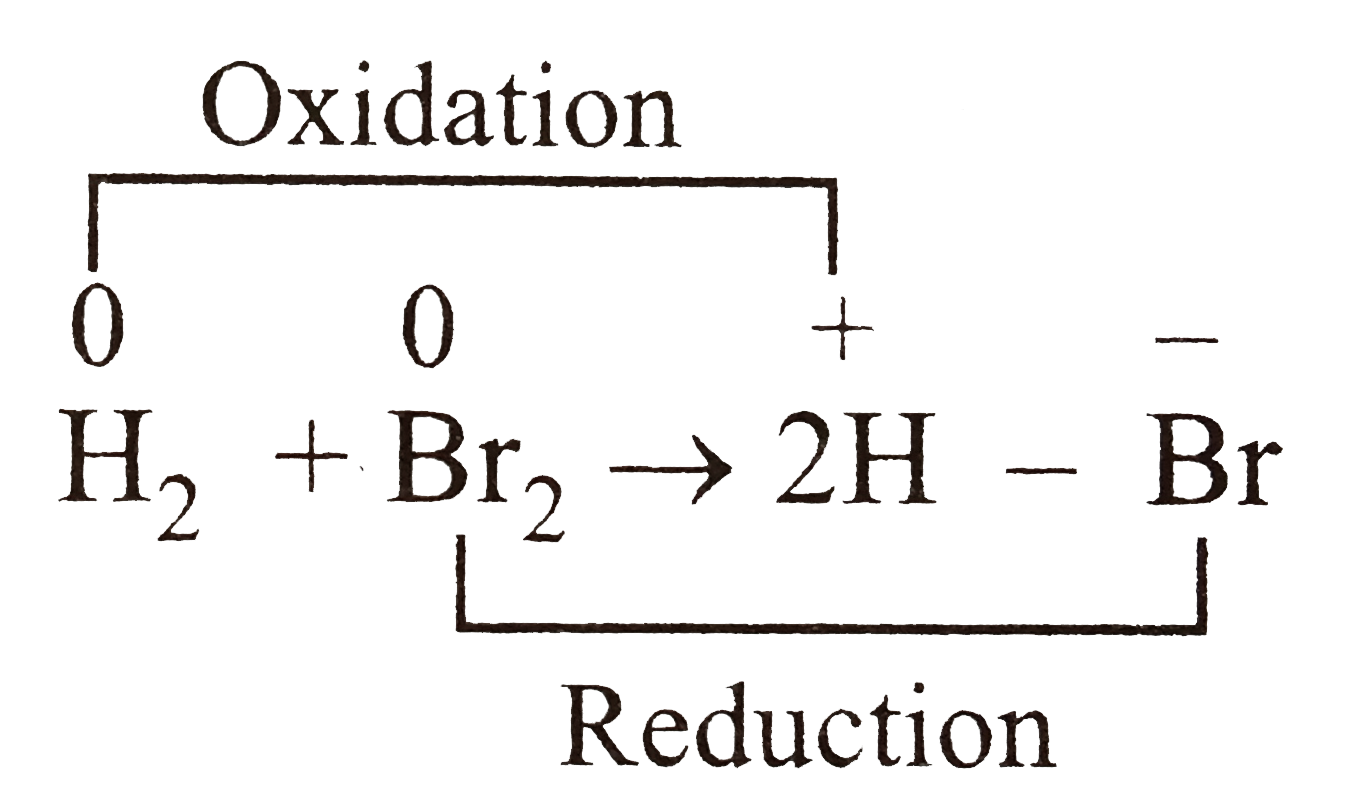
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise Assertion - Reasoning Questions|5 VideosREDOX REACTIONS
A2Z|Exercise Section D - Chapter End Test|29 VideosREDOX REACTIONS
A2Z|Exercise AIPMT/ NEET Questions|16 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-AIIMS Questions
- Following reaction describes the rusting of iron 4Fe+3O(2) rarr 4Fe^...
Text Solution
|
- Identify the correct statement about H(2)O(2)
Text Solution
|
- In C+H(2)O rarr CO+H(2), H(2)O acts as
Text Solution
|
- Which substance is serving as a reducing agent in the following reacti...
Text Solution
|
- HNO(2) acts both as reductant and as oxidant, while HNO(3) acts only a...
Text Solution
|
- Oxidation number if nickel in Ni(CO(4)) is
Text Solution
|
- The oxidation number of carbon in CH(2)Cl(2) is
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- An element which never has a positive oxidation number in any of its c...
Text Solution
|
- If HNO(3) changes into N(2)O, the oxidation number is changed by
Text Solution
|
- The oxidation number of iron in the compound K(4)[Fe(CN)(6)] is
Text Solution
|
- The brown ring complex compound is formulated as [Fe(H(2)O)(5)NO]SO(4)...
Text Solution
|
- The oxidation number of S in Na(2)S(4)O(6) is
Text Solution
|
- Identify the element which can have highest oxidation numbers
Text Solution
|
- What is the net charge on ferrous ion ?
Text Solution
|
- Which of the following reaction involves oxidation reduction?
Text Solution
|
- What is the equivalent weight of phosphoric acid (H(3) PO(4)) accordin...
Text Solution
|
- For decolourisation of 1 "mol of" KMnO(4), the moles of H(2)O(2) requi...
Text Solution
|
- The oxidation number of sulphur in H(2)S(2)O(7) and iron in K(4)Fe(CN)...
Text Solution
|
- MnO(4)^(2-) in neutral aqueous medium is disproportionate to
Text Solution
|