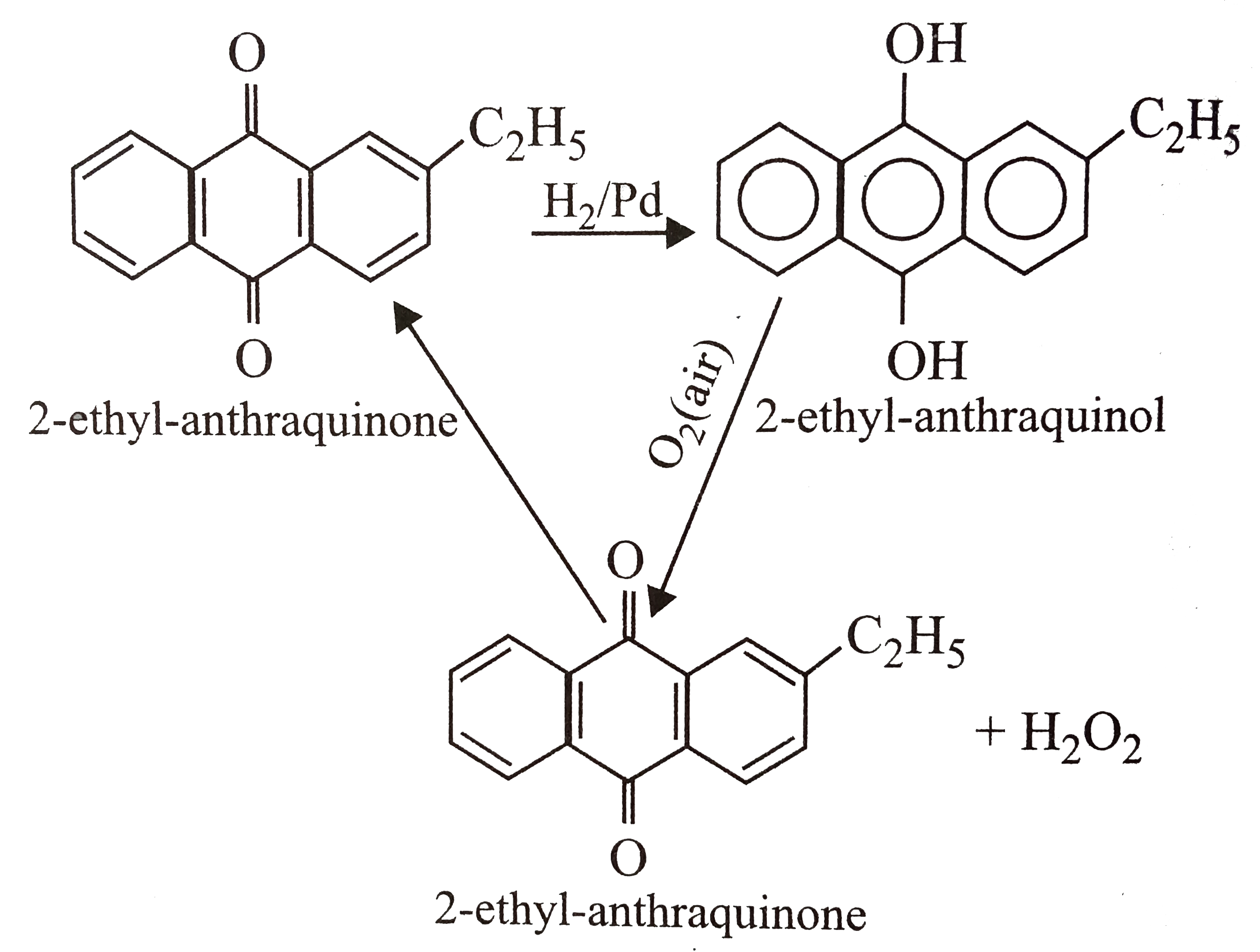
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-HYDROGEN-Hydrogen Peroxide
- H(2)O(2) is used as :
Text Solution
|
- The reaction of H(2)S+H(2)O(2) rarr S+2H(2)O manifests
Text Solution
|
- Hydrogen peroxide is now generally prepared on industrial scale by the
Text Solution
|
- The oxide that gives H(2)O(2) on treatment with a dilute acid is
Text Solution
|
- H(2)O(20 is always stored in black bottles because
Text Solution
|
- H(2)O(2)rarr 2H^(+)+O(2)+2e^(-),E^(@)=-0.68V. This equation represen...
Text Solution
|
- In lab H(2)O(2) is prepared by
Text Solution
|
- The structure of H(2)O(2) is
Text Solution
|
- There is a smaple of 10 volume of hydrogen peroxide solution . Calcula...
Text Solution
|
- Blackened oil painting can be restored into original form by the actio...
Text Solution
|
- In which of the following reaction hydrogen peroxide is a reducing ag...
Text Solution
|
- Commercial 11.2 volume H(2)O(2) solution has a molarity of
Text Solution
|
- Which of the following undergoes reduction with H(2)O(2) in an alkalin...
Text Solution
|
- In the reaction. H(2)S+H(2)O(2) rarr S+2H(2)O
Text Solution
|
- In the reaction, 2FeSO(4) +H(2)SO(4)+H(2)O(2)rarr Fe(2)(SO(4))(3)+2...
Text Solution
|
- in alkaline medium, H(2)O(2) reacts with Fe^(3+) and Mn^(@+) respectiv...
Text Solution
|
- H(2)O(2) can be obtained when following reacts with H(2)SO(4) excepts ...
Text Solution
|
- H(2)O(2)rarr H(2)O+O(2) This represents
Text Solution
|
- Decomposition of H(2)O(2) is accelerated by
Text Solution
|
- H(2)O(20 is manufactured these days
Text Solution
|