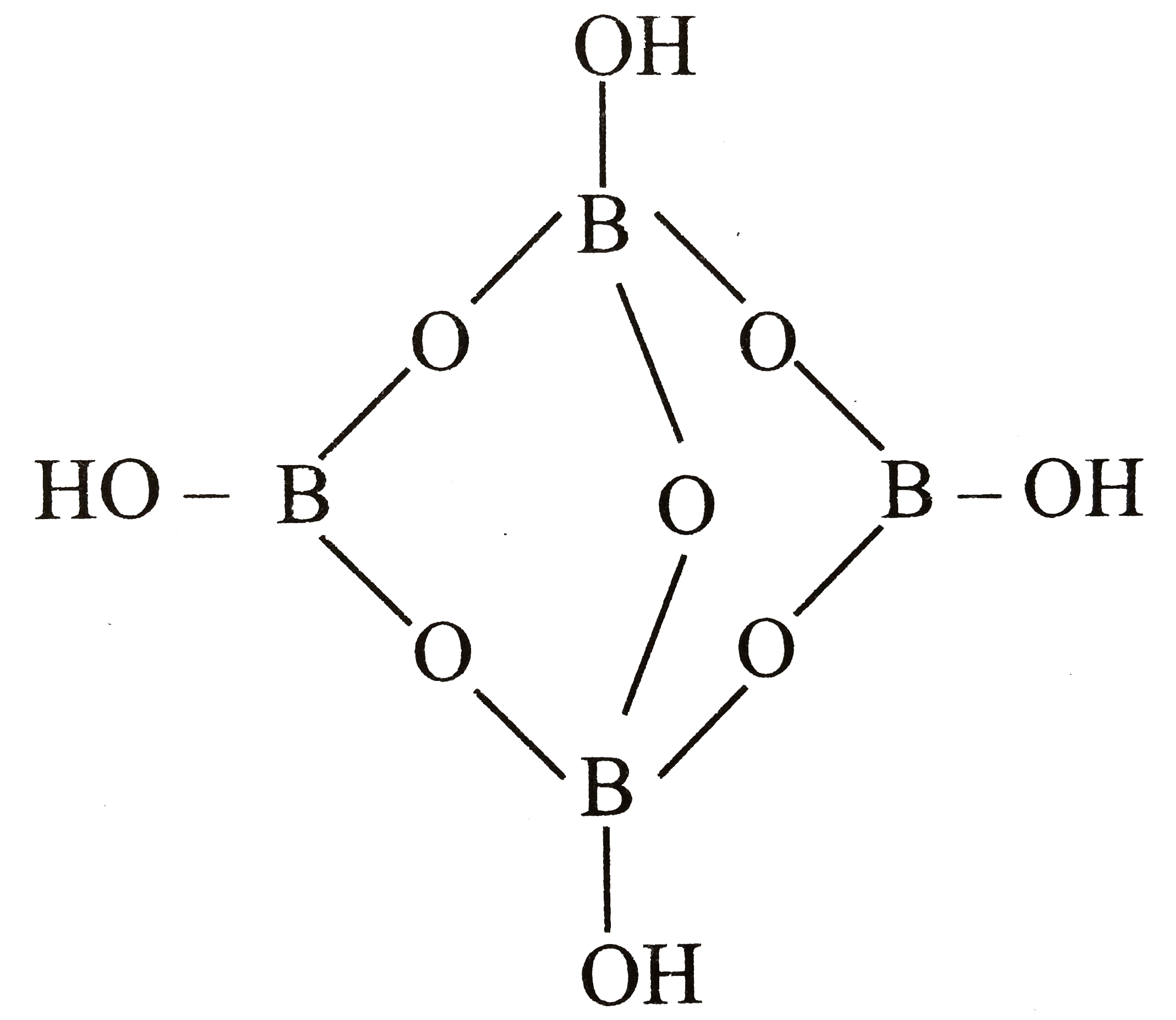
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise General Characterstics, Pysical And Chemical Properties (Group 14)|49 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Compound Of Carbon Family|52 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosMOCK TEST
A2Z|Exercise Mock Test 2|45 VideosREDOX REACTIONS
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( Group 13 -14)-Compound Of Boron Family
- Which one of the following is the correct statement ?
Text Solution
|
- Which of the following statements regarding boric acid is false?
Text Solution
|
- The number of -OH units directly linked to boron atom in Na(2)B(4)O(7)...
Text Solution
|
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- Diborane upon hydrolysis gives
Text Solution
|
- AlCl(3) on hydrolysis gives
Text Solution
|
- Aqueous solution containing 1 mol of borax raects with 2 mol of acids....
Text Solution
|
- An aqueous solution of borax is
Text Solution
|
- Boric acid heated to red hot gives
Text Solution
|
- Which of the following has the minimum heat of dissociation?
Text Solution
|
- There are two H-bridge bonds in diborane molecule because there are
Text Solution
|
- Identify the statement that is not correct as far as structure of dibr...
Text Solution
|
- Boric acid on heating at 150^(@)C gives
Text Solution
|
- Borax bead test is not given by
Text Solution
|
- Boric acid is polymeric due to :
Text Solution
|
- Aluminium chloride exists as a dimer, Al(2)Cl(6) in solid state as wel...
Text Solution
|
- The type (s) of bonds present in diborane is are
Text Solution
|
- In diborane, the banana bond is formed between
Text Solution
|
- which of the following compounds are formed when boron trichloride is ...
Text Solution
|
- Which statement is not true about potas alum?
Text Solution
|