A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise General Characterstics, Pysical And Chemical Properties (Group 14)|49 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Compound Of Carbon Family|52 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosMOCK TEST
A2Z|Exercise Mock Test 2|45 VideosREDOX REACTIONS
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( Group 13 -14)-Compound Of Boron Family
- Aqueous solution containing 1 mol of borax raects with 2 mol of acids....
Text Solution
|
- An aqueous solution of borax is
Text Solution
|
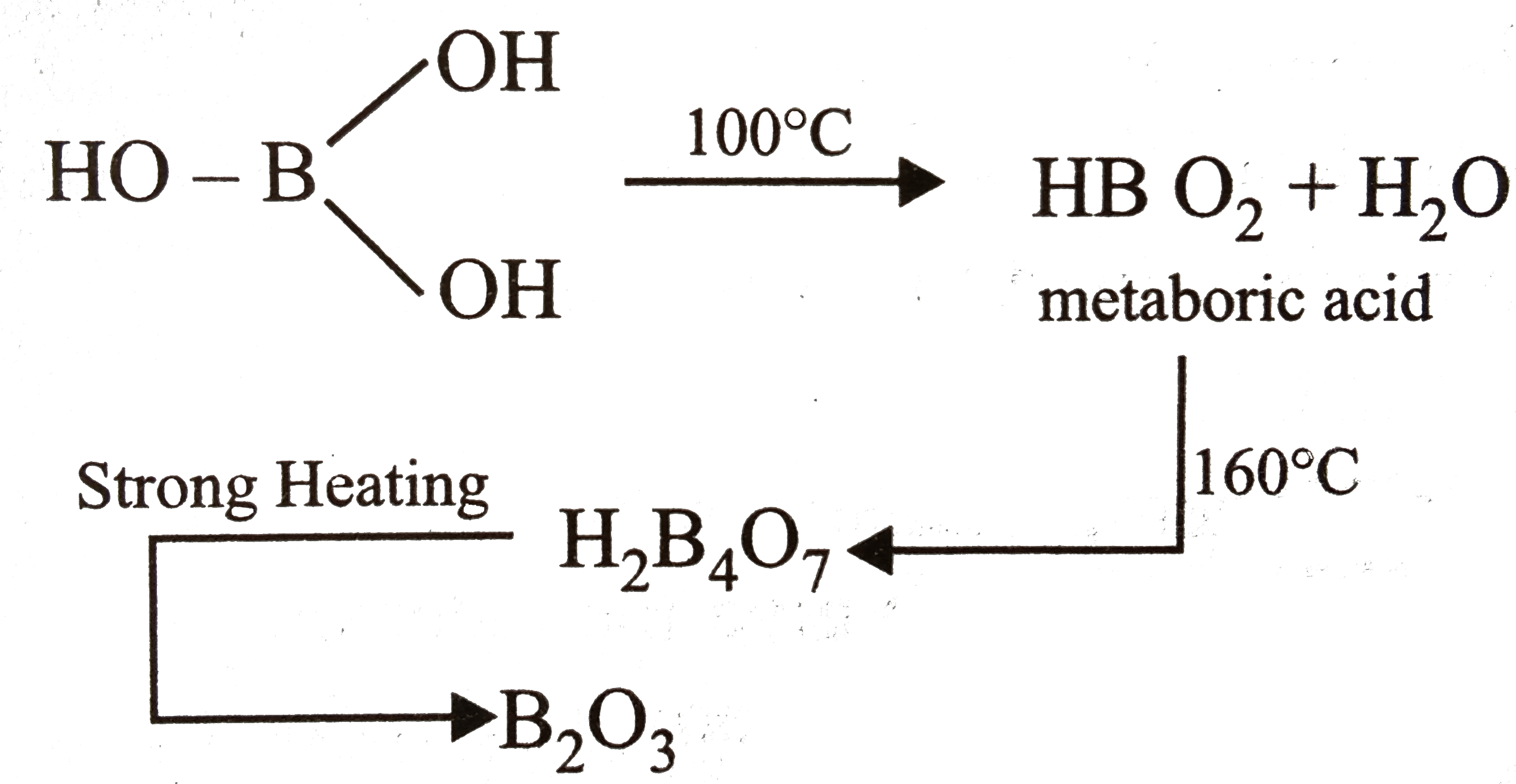
- Boric acid heated to red hot gives
Text Solution
|
- Which of the following has the minimum heat of dissociation?
Text Solution
|
- There are two H-bridge bonds in diborane molecule because there are
Text Solution
|
- Identify the statement that is not correct as far as structure of dibr...
Text Solution
|
- Boric acid on heating at 150^(@)C gives
Text Solution
|
- Borax bead test is not given by
Text Solution
|
- Boric acid is polymeric due to :
Text Solution
|
- Aluminium chloride exists as a dimer, Al(2)Cl(6) in solid state as wel...
Text Solution
|
- The type (s) of bonds present in diborane is are
Text Solution
|
- In diborane, the banana bond is formed between
Text Solution
|
- which of the following compounds are formed when boron trichloride is ...
Text Solution
|
- Which statement is not true about potas alum?
Text Solution
|
- Heating an aqueous solution of aluminium chloride to dryness will give
Text Solution
|
- Boron cannot from which one of the following anions?
Text Solution
|
- When excess of NaOH solution is added in potash alum the product is.
Text Solution
|
- H(3)BO(3) ionises in water gas
Text Solution
|
- The correct formula of borax is
Text Solution
|
- Reactivity of borazole is greater than that of benzene because
Text Solution
|