A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise General Characterstics, Pysical And Chemical Properties (Group 14)|49 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Compound Of Carbon Family|52 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosMOCK TEST
A2Z|Exercise Mock Test 2|45 VideosREDOX REACTIONS
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( Group 13 -14)-Compound Of Boron Family
- The correct formula of borax is
Text Solution
|
- Reactivity of borazole is greater than that of benzene because
Text Solution
|
- Diborane does not undergo cleavage reaction with
Text Solution
|
- Boric acid is not used:
Text Solution
|
- In the reaction: BF(3)+3LiBH(4)to3LiF+X, X is
Text Solution
|
- Which one of the following statement regarding BF(3) is not correct?
Text Solution
|
- Diborane belongs to the
Text Solution
|
- In diborane
Text Solution
|
- Boron nitride is isoelectronic with
Text Solution
|
- which of the following general fomulae representation alum?
Text Solution
|
- Alane is chemically
Text Solution
|
- Pick up the wrong statement.
Text Solution
|
- The structure of aluminium bromide is best represented as
Text Solution
|
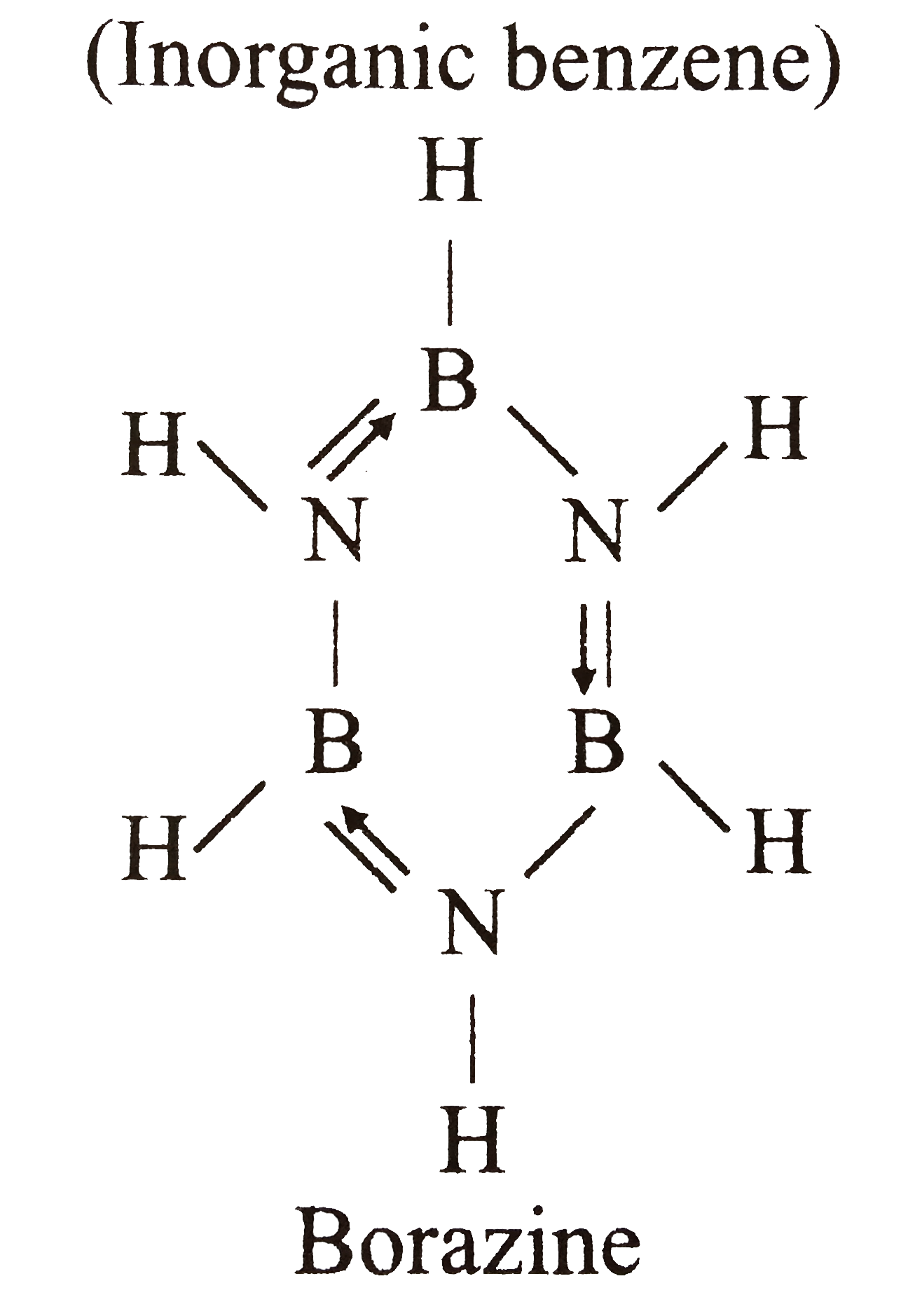
- Which of the following is called inorganic benzene?
Text Solution
|
- A certain salt (X) gives the following tests : (a) Its aqueous solut...
Text Solution
|
- When alumina is heated with carbon in the atmosphere of nitrogen then ...
Text Solution
|
- The form of BN which is hard as diamond is
Text Solution
|
- In presence of aluminium chloride, diborane reacts with dry hydrogen c...
Text Solution
|
- Diborane is a Lewis acid forming addition compound B(2)H(6).2NH(3) wit...
Text Solution
|
- Borazole is obtained by reacting B(2)H(6) with:
Text Solution
|