A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Assertion - Reasoning Questions|4 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise AIPMT/ NEET Questions|15 VideosMOCK TEST
A2Z|Exercise Mock Test 2|45 VideosREDOX REACTIONS
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( Group 13 -14)-AIIMS Questions
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- The liquified metal expanding on solidification is :
Text Solution
|
- Which of the following is acidic in nature ?
Text Solution
|
- Number of water molecules in Mohr's salt is
Text Solution
|
- Bauxite is purified by
Text Solution
|
- In electrolysis of aluminium oxide which of the following is added to ...
Text Solution
|
- The purification of alumina is called
Text Solution
|
- In Hall's process, the main reagent is mixed with
Text Solution
|
- Assertion: [Al(NH(3))(6)]^(3+) does not exist in aqueous solution. R...
Text Solution
|
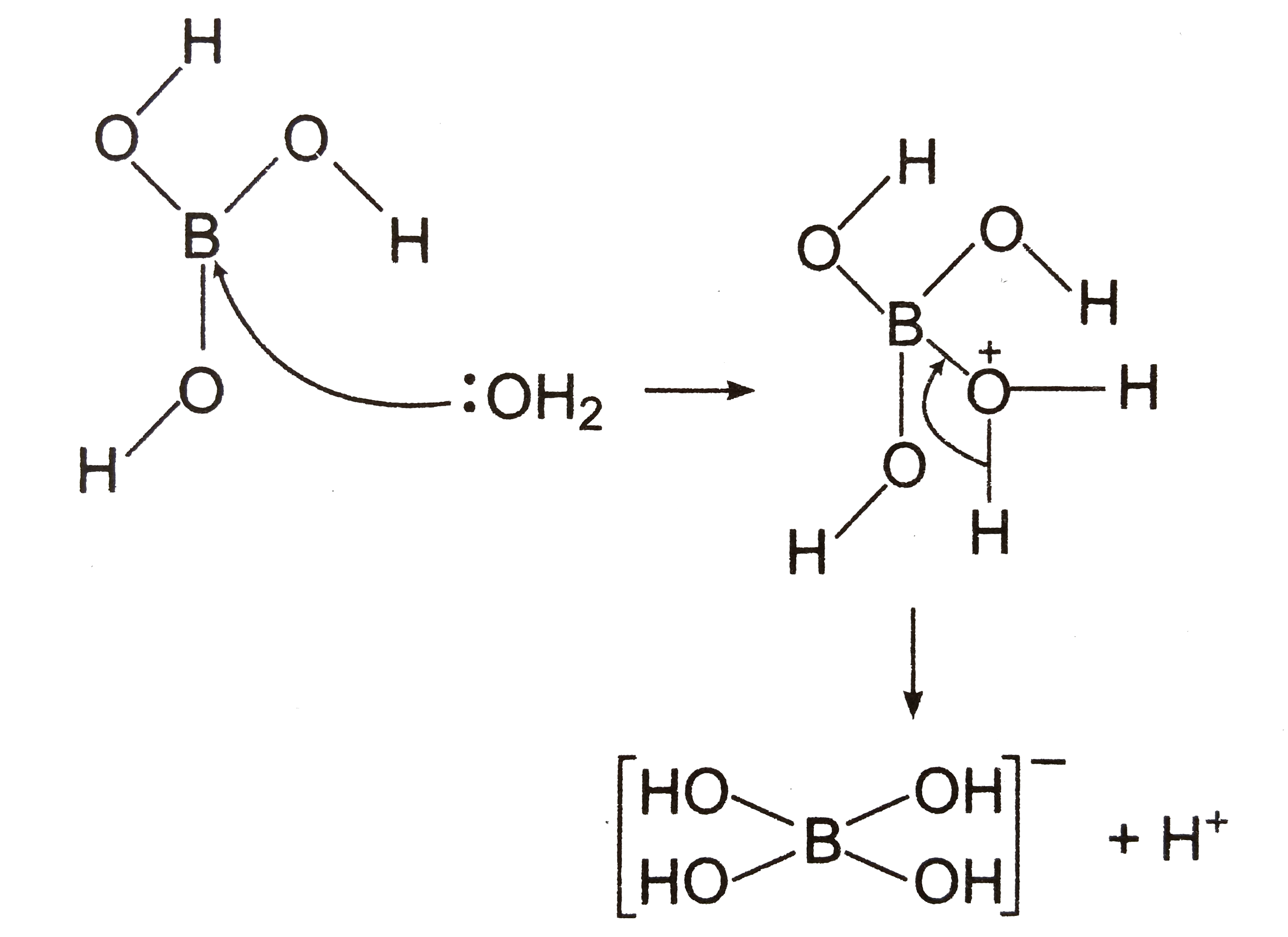
- Statement: In water, orthoboric acid behaves as a weak monobasic acid....
Text Solution
|
- Assertion: Boron always froms covalent bond. Reason: The small size ...
Text Solution
|
- Which of the following gives propyne on hydrolysis ?
Text Solution
|
- Which of the following is the correct statement for red lead?
Text Solution
|
- In laboratory silicon can be prepared by the reaction
Text Solution
|
- Which of the following cuts ultraviolet rays?
Text Solution
|
- Lapis-Lazuli' is a blue coloured precious stone. It is mineral of the ...
Text Solution
|
- Lead pipes are corroded quickly by
Text Solution
|
- Which gas is liberated when Al(4)C(3) is hydrolysed ?
Text Solution
|
- Which of the following attacks glass?
Text Solution
|