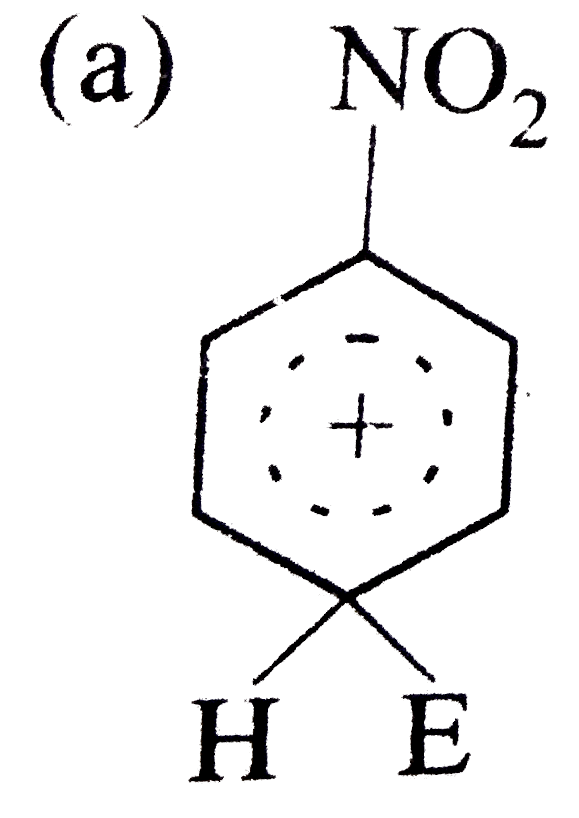
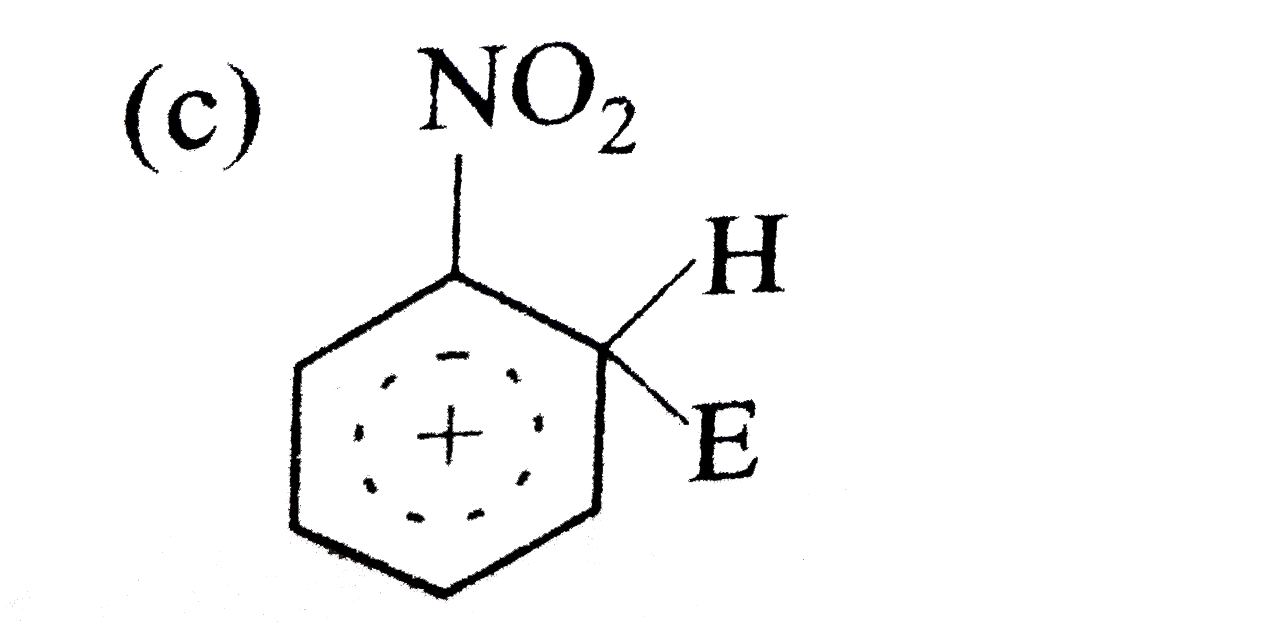
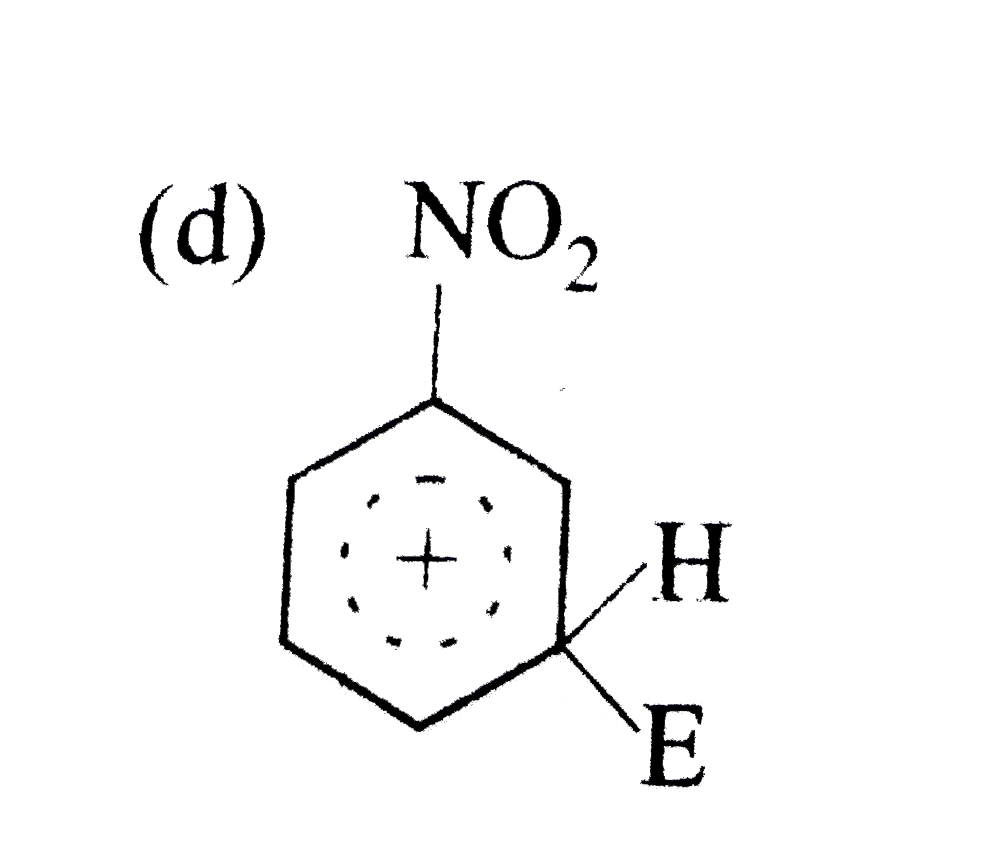
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Structure Isomerism|28 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Tautomerism|16 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Acid And Basic Strength|29 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosSTATES OF MATTER
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY-Bond Fission, Reagents, Reactive Intermediates And Their Stability
- Which of the following is not a nucleophile?
Text Solution
|
- The stability order of following carbocation is
Text Solution
|
- The electrophile, E^((o+)) attacks the benzene ring to generate the in...
Text Solution
|
- The compound which would give the most stable carbocation on dehydrati...
Text Solution
|
- Which of the following is the rearranged more stable carbocation of th...
Text Solution
|
- Which of the following is the rearranged more stable carbocation of th...
Text Solution
|
- Which of the following is the least stable carbanion?
Text Solution
|
- Arrange the following carbocation in decreasing order of stability.
Text Solution
|
- Arrange the following carbocation in decreasing order of stability.
Text Solution
|
- Among the following, the paramagnetic species is:
Text Solution
|
- The increasing order of stability of the following free radicals is:
Text Solution
|
- The hybrid state of positively charged carbon in vinyl (CH2 = CH^+ ) c...
Text Solution
|
- The stability of given free radicals in decreasing order is (i) CH(3...
Text Solution
|
- Which of the following is the correct order of stability of free radic...
Text Solution
|
- In the following carbocations, the stability order is:
Text Solution
|
- The decreasing order of stability of the following cations is unders...
Text Solution
|
- Which of the following is most stable carbocation?
Text Solution
|
- Which of the following is most stable carbocation?
Text Solution
|
- The most stable carbocation is:
Text Solution
|
- Arrange the following carbanions in decreasing order of stability: ...
Text Solution
|