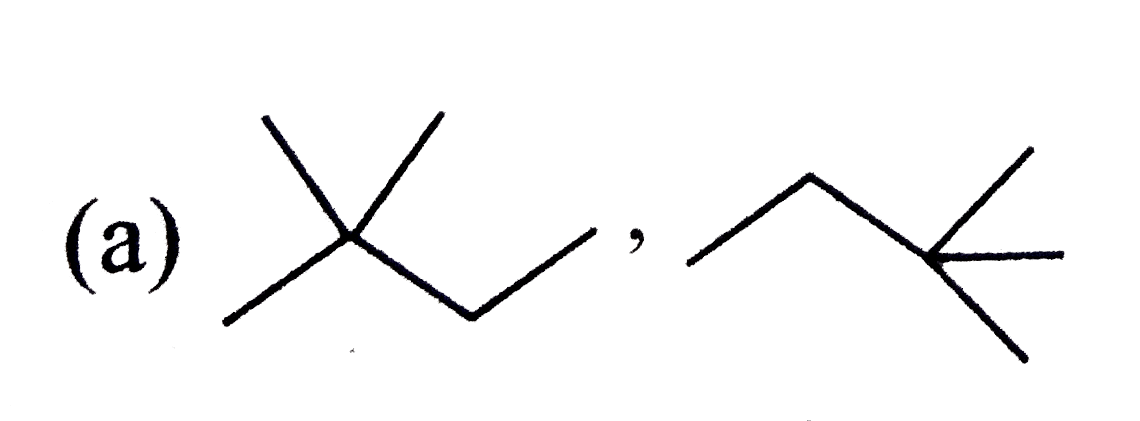
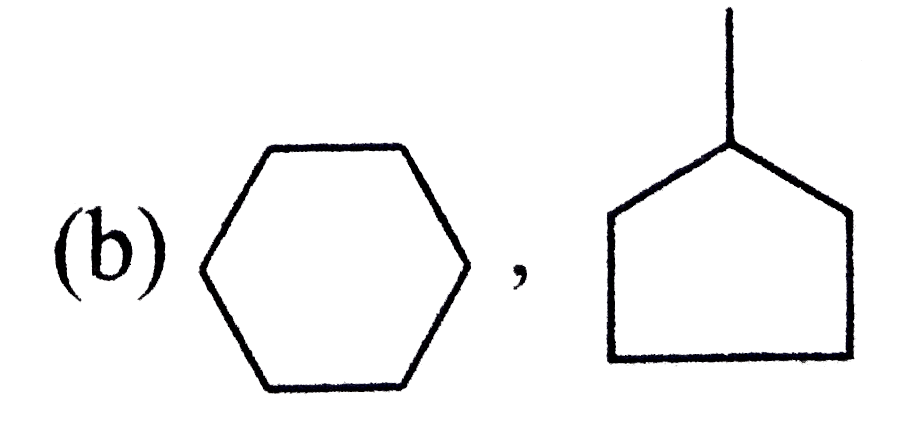
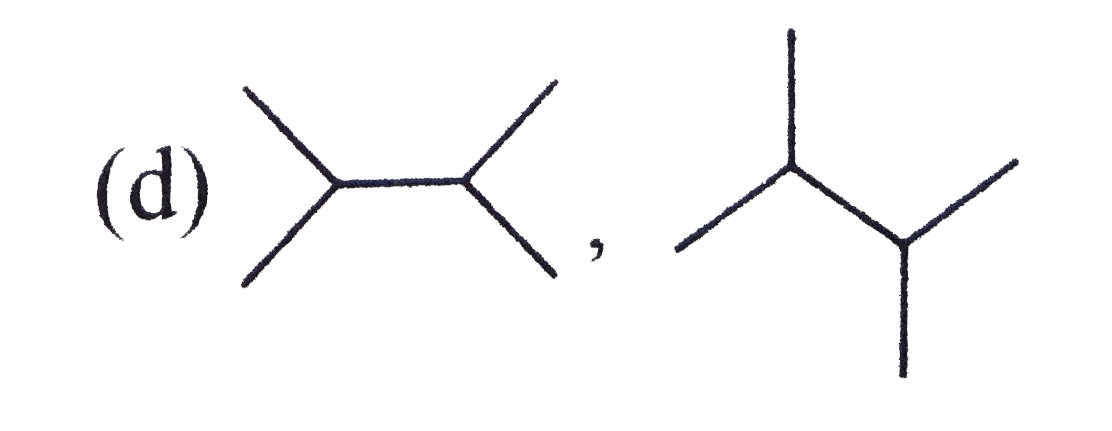
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Tautomerism|16 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Geometrical Isomerism|47 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Bond Fission, Reagents, Reactive Intermediates And Their Stability|31 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosSTATES OF MATTER
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY-Structure Isomerism
- What is the correct relationship between the following compounds? CH...
Text Solution
|
- Which of the following statements regarding ethanoic acid and methyl m...
Text Solution
|
- Which of the following pairs is the chain isomer?
Text Solution
|
- Which of the following statements concerning 3,4-dibromo-1-pentane and...
Text Solution
|
- What is the relation between 3-Ethylpentane and 3-Methylhexane?
Text Solution
|
- How many structural isomers C(4)H(8) have?
Text Solution
|
- Relation between the above compound is:
Text Solution
|
- Constitutional isomerism is possible in alkanes only if the number car...
Text Solution
|
- Total number of position isomers of dimethyl cyclohexane.
Text Solution
|
- How many structural isomers (aldehyde+ketone) are possible for C(5)H(1...
Text Solution
|
- Which of the following shows functional isomerism?
Text Solution
|
- Organic compound containing carbon, hydrogen and nitrogen, can be eith...
Text Solution
|
- Which type of isomerism is observed between I and II? underset((I))(...
Text Solution
|
- How many different structural isomers exist for C(3)H(6)O in which no ...
Text Solution
|
- The isomer of diethyl ether is
Text Solution
|
- How many structrural isomer exist for C(7)H(8)O where each isomer con...
Text Solution
|
- How many structrual isomers of tertiary amines corresponding to molecu...
Text Solution
|
- What is wrong about enantiomers of 2-chloropropanoic acid?
Text Solution
|
- How many structural isomers are possible for compound contaning C,H an...
Text Solution
|
- Which of the following compound are structural isomers of C(6)H(10)O? ...
Text Solution
|