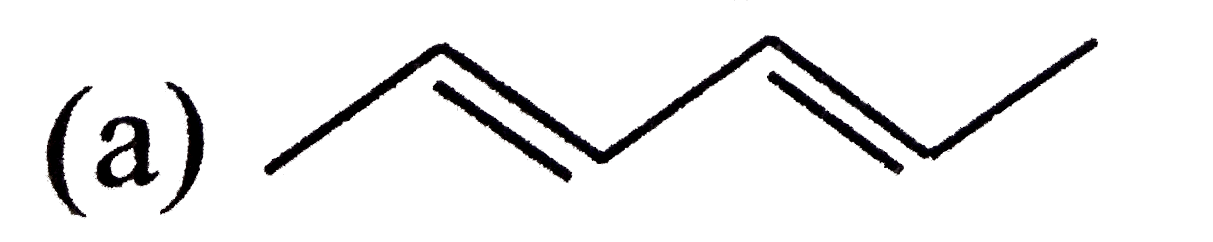
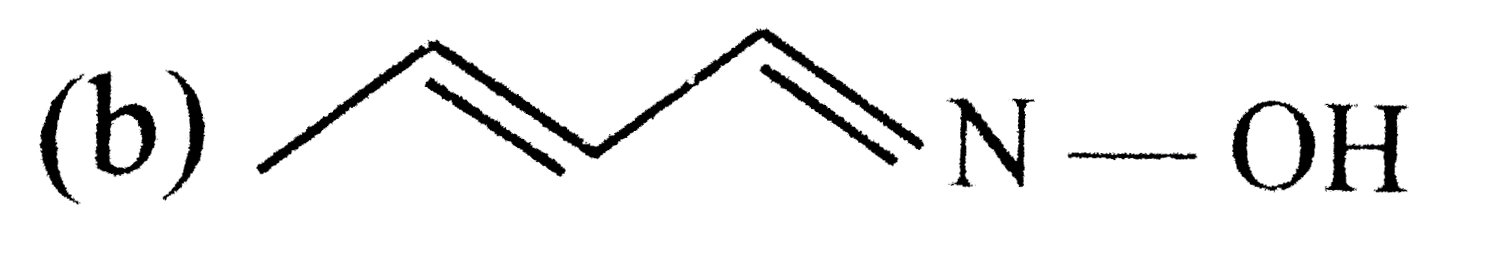
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Optical Isomerism|53 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Confirmational Isomerism|25 VideosSOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY
A2Z|Exercise Tautomerism|16 VideosSOME BASIC CONCEPTS OF CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosSTATES OF MATTER
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOME BASIC PRINCIPALS OF ORGANIC CHEMISTRY-Geometrical Isomerism
- How many cyclic isomers exist (structural and geometrical only) for C(...
Text Solution
|
- Which of the following carbonyls has four different enol isomers ?
Text Solution
|
- CH(3)-underset(Cl-C-Br)underset(||)C-Cl is
Text Solution
|
- Which compound below has four stereoisomers?
Text Solution
|
- How many cyclic isomers (structrural and geometrical only) exist for ...
Text Solution
|
- Geometrical isomers differ in:
Text Solution
|
- The correct stereochemical name of
Text Solution
|
- Which compound can show geometrical isomerism?
Text Solution
|
- Which of the following not show cis-trans isomerism?
Text Solution
|
- Which is a pair of geometrical isomers is ?
Text Solution
|
- The alkene that exhibits geometrical isomerism is
Text Solution
|
- Which of the following does not show geometrical isomerism?
Text Solution
|
- The 'Z'-isomer is
Text Solution
|
- False statement is
Text Solution
|
- Which of the following compound will exhibit cis-trans isomerism?
Text Solution
|
- The number of geometrical isomers for CH(3)-CH=CH-CH=CH-CH=CH(2) is
Text Solution
|
- Which of the following compound can show geometrical isomerism?
Text Solution
|
- The number of isomers for the compound with molecular formula C(2)BrCl...
Text Solution
|
- Which will show geometrical isomerism?
Text Solution
|
- the correct stereochemical formula of trans-3-chloro-1-phenylbut-1-ene...
Text Solution
|