A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET MAJOR TEST (COACHING)-DRILL TEST 1-PHYSICS
- A body starts from rest from the origin with an acceleration of 3 m//s...
Text Solution
|
- A weight of 200 kg is suspended by vertical wire of length 600.5 cm. T...
Text Solution
|
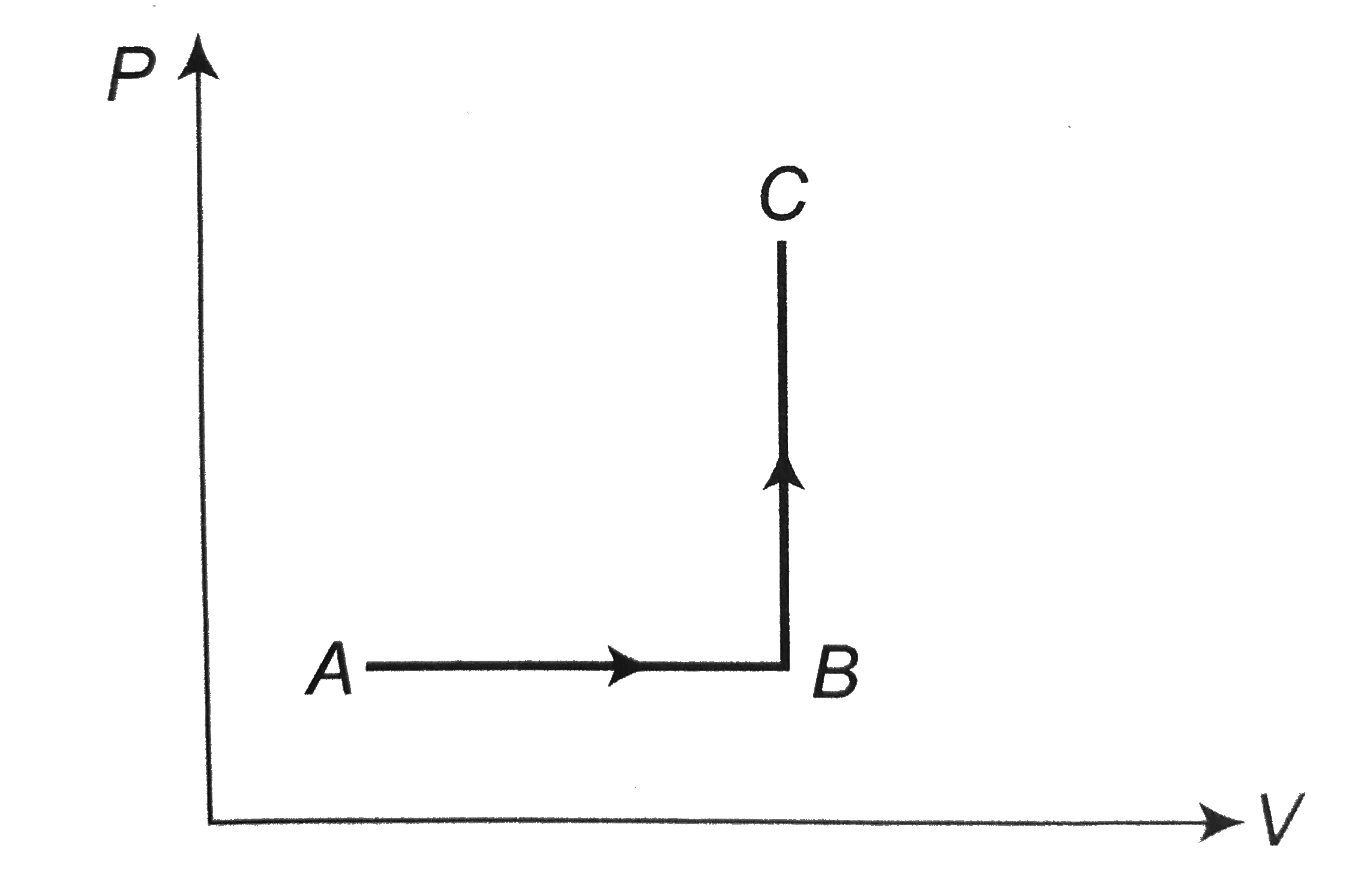
- The P - V diagram of a system undergoing thermodynamic transformation ...
Text Solution
|
- The length of a spring is l and its force constant is k . When a weigh...
Text Solution
|
- A source sound is moving with constant velocity of 20m//s emitting a n...
Text Solution
|
- A body A moves with a uniform acceleration a and zero initial velocity...
Text Solution
|
- A 2 m long rod of radius 1 cm which is fixed from one end is given a t...
Text Solution
|
- The temperature of argon, kept in a vessel, is raised by 1^(@)C at a c...
Text Solution
|
- In figure S(1) andS(1) are identical springs. The oscillation frequenc...
Text Solution
|
- While measuring acceleration due to gravity by simpe pendulum a studen...
Text Solution
|
- If the velocity of a particle is given by v=(180-16x)^((1)/(2))(m)/(s)...
Text Solution
|
- The radius of a soap bubble is increased from (1)/(sqrtpi) cm to (2)/(...
Text Solution
|
- How many degress of freedom have the gas molecules, if under standard ...
Text Solution
|
- Two monoatomic ideal gases 1 and 2 of molecular masses m^(1) and m^(...
Text Solution
|
- The volume of a sphere is .176 m^3 What will be the volume of 25 such ...
Text Solution
|
- A ball is projected upwards from a height h above the surface of the e...
Text Solution
|
- A tank 5 m high is half filled with water and then is filled to top wi...
Text Solution
|
- When p calories of heat is given to a body, it absorbs q calories, t...
Text Solution
|
- A wave is represented by the equetion y=7 sin(7pi t-0.04pix+(pi)/(3))...
Text Solution
|
- At t = 0 a 100gm ball is thrown upwards with initial speed v(0) = 2 m/...
Text Solution
|