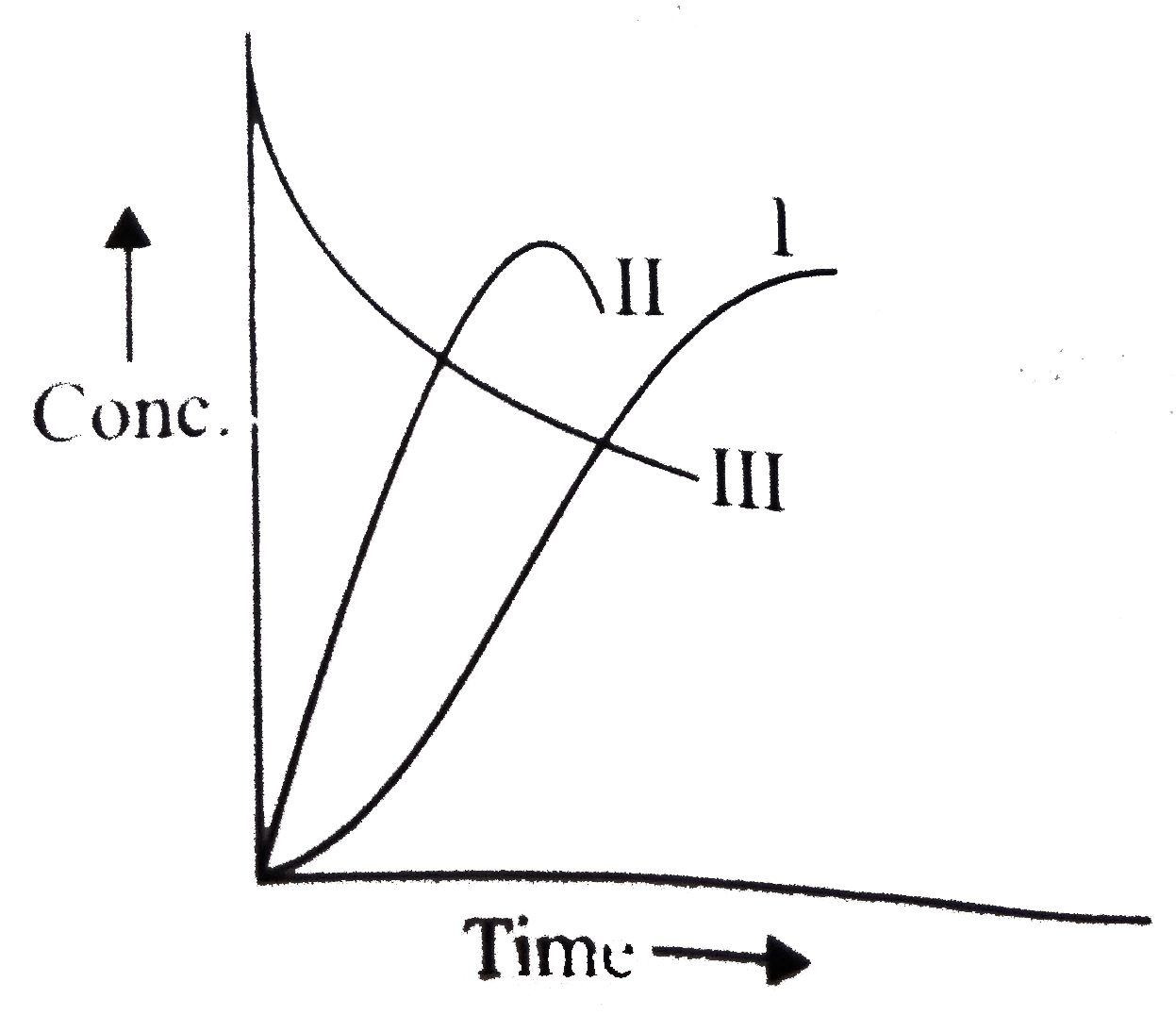
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
A2Z|Exercise Section B - Assertion Reasoning|21 VideosCHEMICAL KINETICS
A2Z|Exercise AIPMT/NEET Questions|43 VideosCHEMICAL KINETICS
A2Z|Exercise Arrhenius Equation, Effect Of Temprature And Effect Of Catalysts|35 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL KINETICS-Parallel Reaction, Consecutive Reaction, Special Cases Of First-Order Reactions
- The acid catalysed ionisation of gamma-hydroxy butyric acid proceeds a...
Text Solution
|
- Two reactions A rarr products and B rarr products have rate constants ...
Text Solution
|
- A simple mechanism for enzyme-catalyzed reaction is given by the folll...
Text Solution
|
- For a reaction, A rarr B+C, it was found that at the end of 10 minutes...
Text Solution
|
- Consider the reaction, Cl(2)(aq) + H(2)S(aq) rarr S(s) + 2H^(+) (aq)...
Text Solution
|
- For a Ist order decomposition of A as given Therefore rate const...
Text Solution
|
- For the consecutive unimolecular-type first-order reaction A overset(k...
Text Solution
|
- For a reaction ,(d[X])/(d t) is ewqual to
Text Solution
|
- Choose the correct set of identification.
Text Solution
|
- The mechanism of the reaction: A + 2B + C rarr D is (step 1) (fast) ...
Text Solution
|
- Catalyst decomposition of hydrogen peroxide is a….. Order reaction
Text Solution
|
- In the reaction NH(4)NO(2)(aq.) rarr N(2)(g) + 2H(2)O(l) the volume of...
Text Solution
|
- The half life of decompoistion of N(2)O(5) is a first order reaction r...
Text Solution
|
- Half life id independent of the concentration of A. After 10 mi n volu...
Text Solution
|
- For the consecutive unimolecular first order reaction A overset(k(1))r...
Text Solution
|
- A first order homogeneous reaction of the type X rarrY rarr Z (consecu...
Text Solution
|
- Consider the elementary reaction sequence shown in figure. Which of th...
Text Solution
|
- A hypothetical reaction A(2) + B(2) rarr 2AB follows the mechanism as ...
Text Solution
|
- For a gaseous reaction, following data is given: ArarrB, k(1)= 10^(1...
Text Solution
|
- H(2)O and O atom react in upper atmosphere bimolecularly to form two O...
Text Solution
|