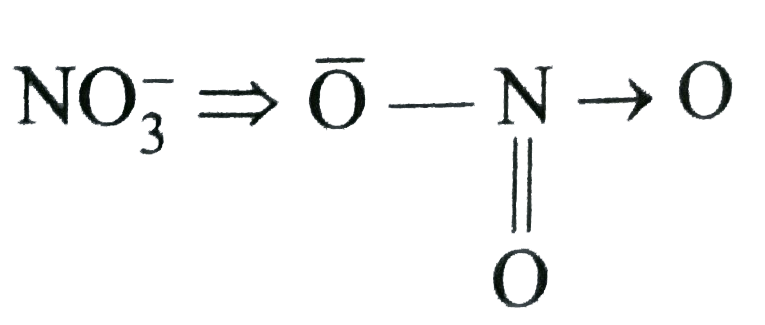A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIIMS Questions|25 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Assertion-Reasoning Questions|14 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section B - Assertion Reasoning|35 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-AIPMT/NEET Questions
- Oxidation number of As in H(2)"As"O(4)^(-) is
Text Solution
|
- Which of the following combines with Fe^(2+) ions to form brown comple...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pair and lone pair of electrons o...
Text Solution
|
- Which of the following statements is wrong?
Text Solution
|
- Which of the following statements is not valid for oxo-acids of phosho...
Text Solution
|
- Nitrogen dioxide and sulphur dioxide have some properties in common. W...
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to:
Text Solution
|
- When copper is heated with conc. HNO(3) it produces?
Text Solution
|
- Which of the following statement is correct for the given acids?
Text Solution
|
- The product obtained a result of a reaction of nitrogen with CaC(2) is
Text Solution
|
- Bleaching action of SO(2) is due to
Text Solution
|
- In the reaction 2Ag+2H(2)SO(4)rarrAg(2)SO(4)+2H(2)O+SO(2),H(2)SO(40act...
Text Solution
|
- By passing H(2)S in acidified KMnO(4) solution we get
Text Solution
|
- Which one of the gas dissolves in H(2)SO(4) to give oleum?
Text Solution
|
- When SO(4) is passed through acidified K(2)Cr(2)O(7) solution
Text Solution
|
- KO(2)+CO(2)rarr?("gas")
Text Solution
|
- Hypo is used in photography to
Text Solution
|
- There is no S-S bond in
Text Solution
|
- Which of the following is not a chalcogen?
Text Solution
|
- Oxygen molecule exhibits
Text Solution
|