A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIIMS Questions|25 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Assertion-Reasoning Questions|14 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section B - Assertion Reasoning|35 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-AIPMT/NEET Questions
- The variation of the boiling points of the hydrogen halides is in the ...
Text Solution
|
- Among the following, the correct order of acidity is:
Text Solution
|
- Which one of the following orders is correct for the bond dissociation...
Text Solution
|
- Among the fluorides below, the one which does not exist is
Text Solution
|
- Which one of the following configurations represents a noble gas?
Text Solution
|
- Which of the following is monoatomic?
Text Solution
|
- The noble gas which forms maximum number of compound is
Text Solution
|
- The electronic configuration of neon is
Text Solution
|
- The inert gases are
Text Solution
|
- What is the total number of electron present in the last orbit of argo...
Text Solution
|
- Which of the following gases is//are called rare gas?
Text Solution
|
- Which is planar molecule ?
Text Solution
|
- Which noble gas is more soluble in water ?
Text Solution
|
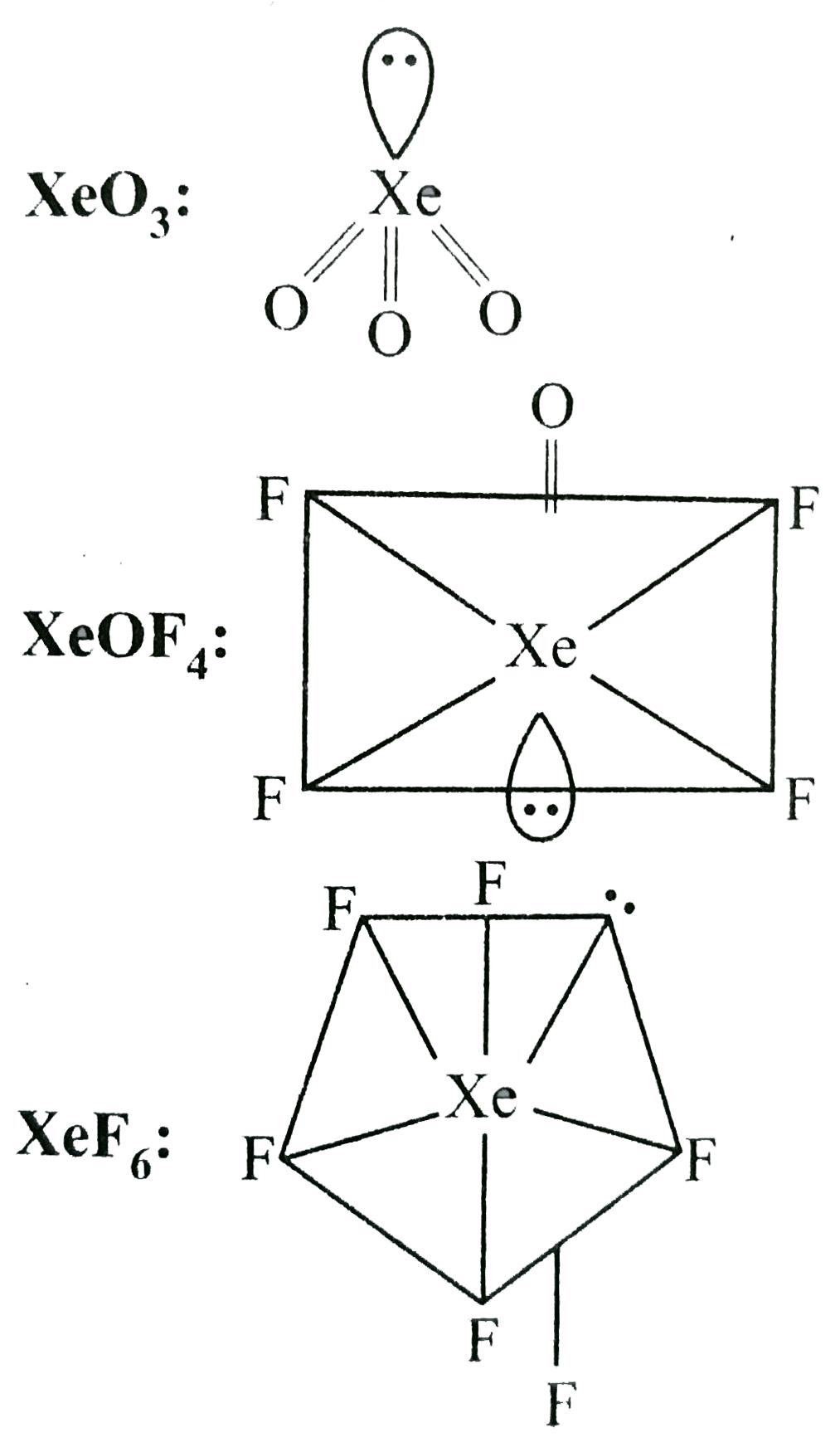
- Among the following molecules, (i)XeO(3)(ii)XeOF(4)(iii)XeF(6) those h...
Text Solution
|
- In which pair of ions both the species contains S - S bond?
Text Solution
|
- Which of the following statements is not ture for halogens?
Text Solution
|
- Which ordering of compounds is according to the decreasing order of th...
Text Solution
|
- A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with c...
Text Solution
|
- The number of lone pairs of electrons present on the central atom of C...
Text Solution
|
- Which oxide of nitrogen is not a common pollutant introduced into the ...
Text Solution
|