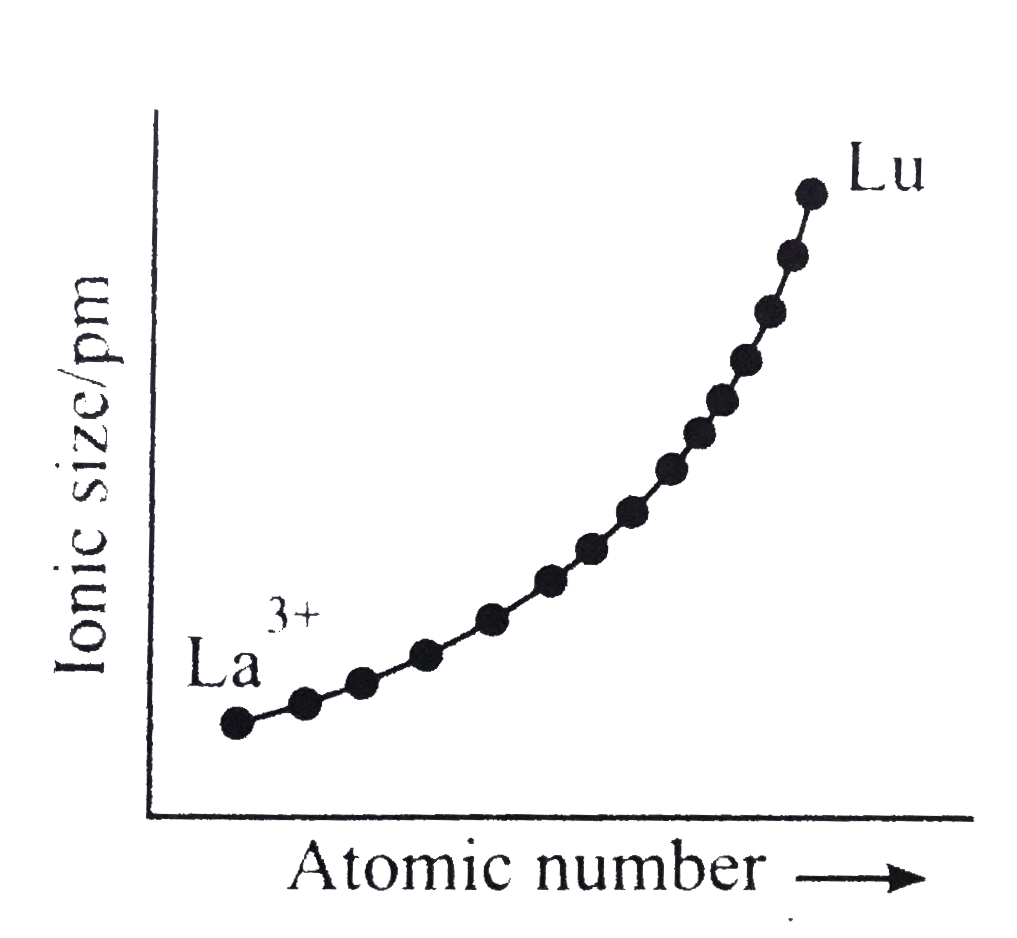
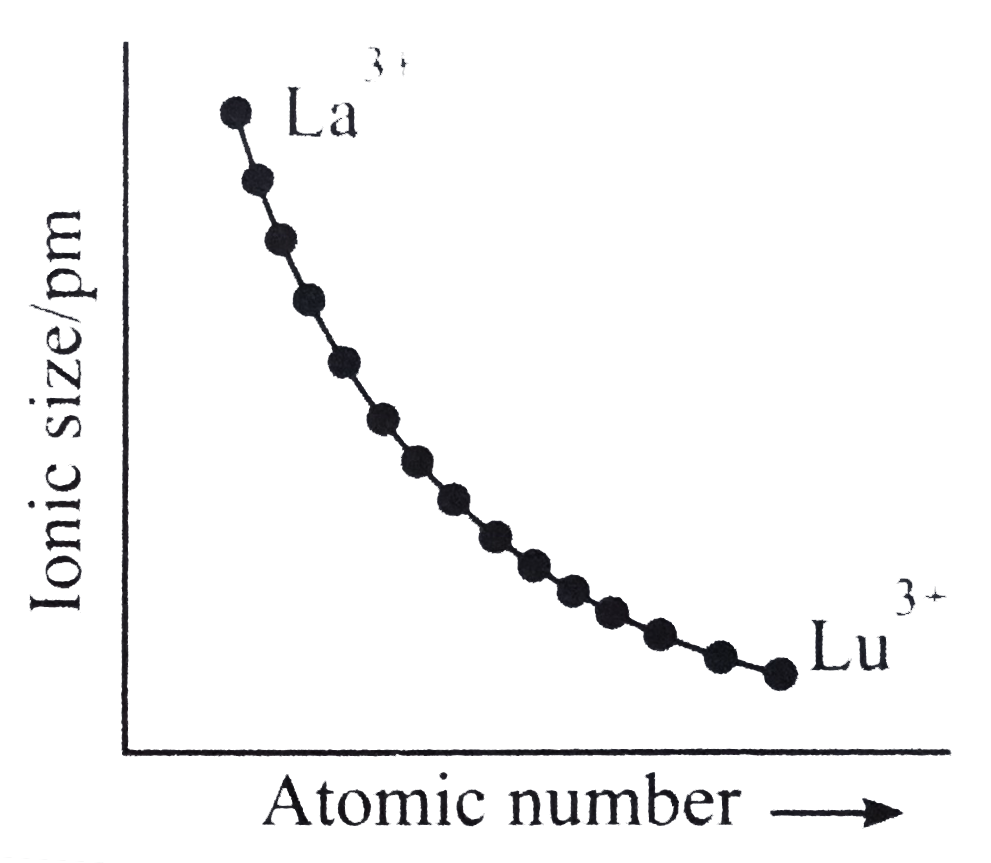
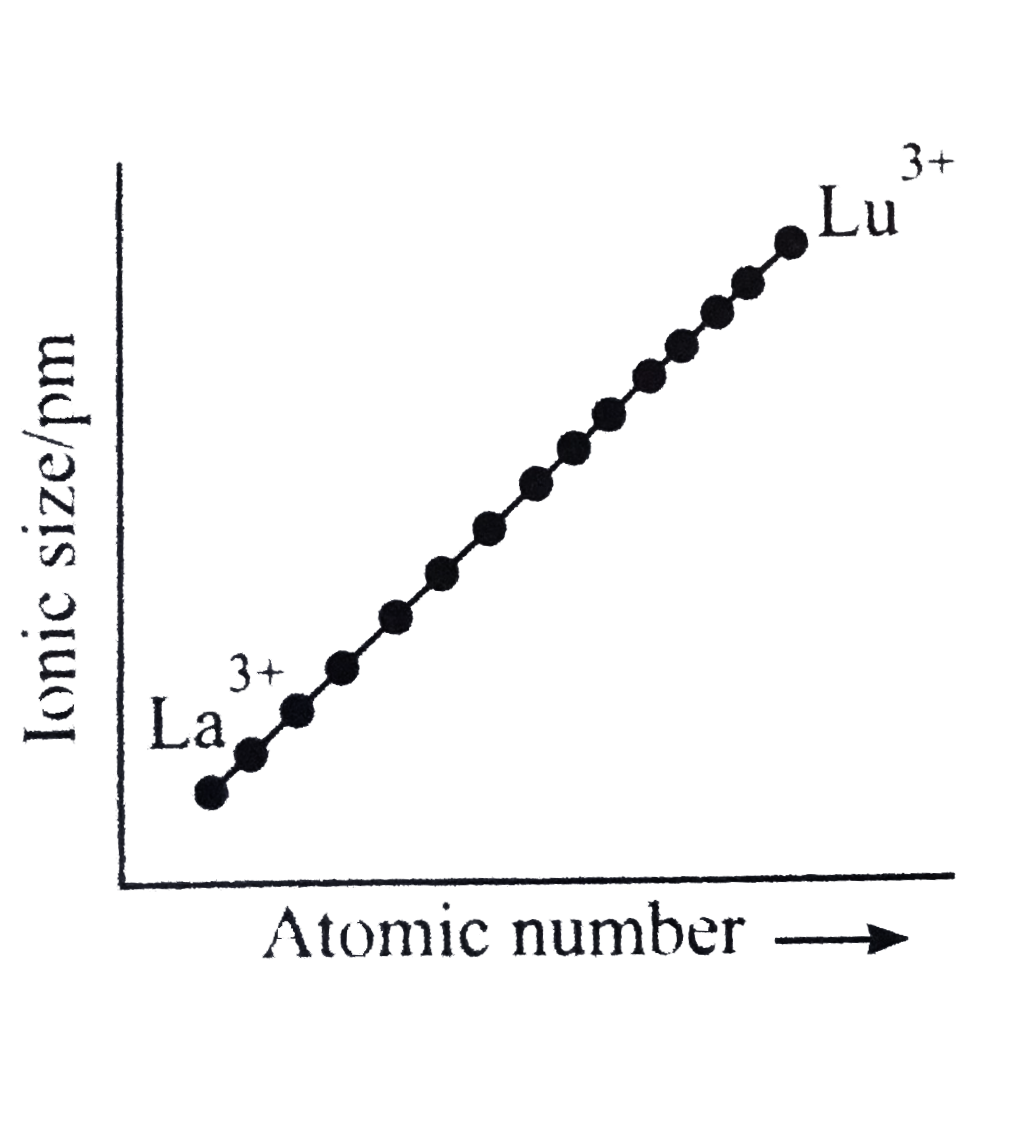
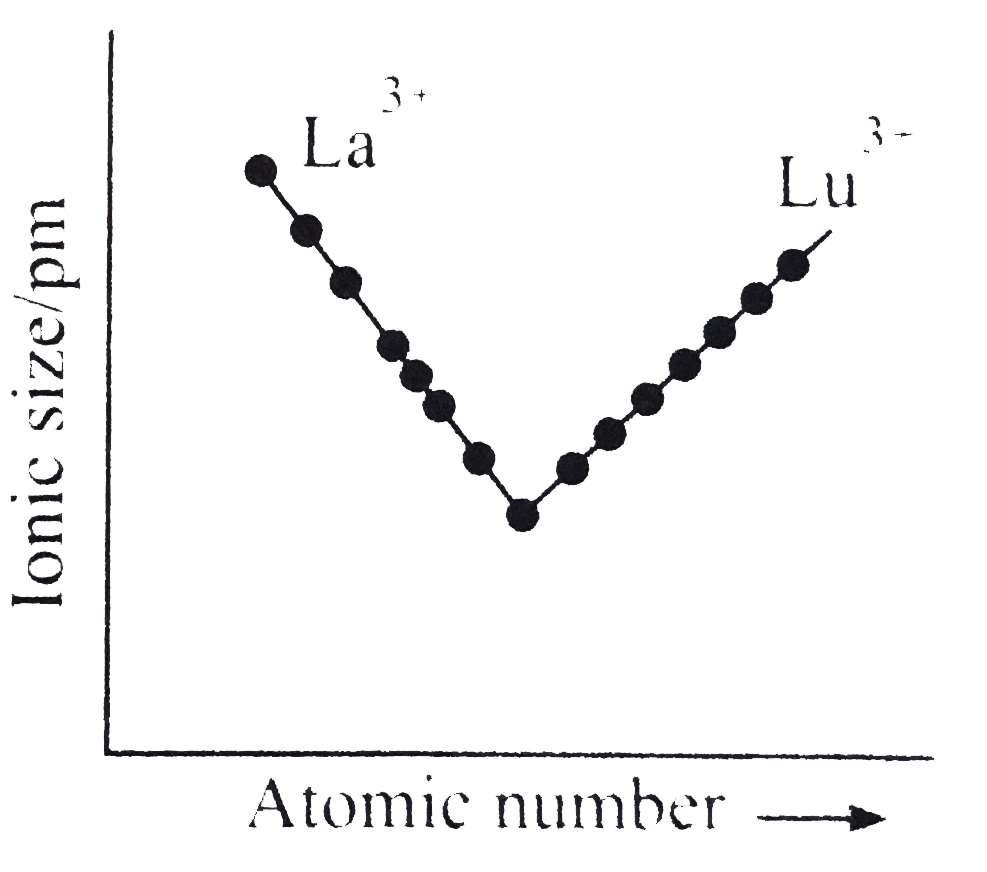
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-THE D AND F BLOCK ELEMENTS-Lanthanides And Actinides
- Across the lanthanide series, the basicity of the lanthanoid hydroxide...
Text Solution
|
- Lanthanides and actinides resemble in
Text Solution
|
- Lanthanides contraction cause.
Text Solution
|
- The speration of lanthanides by ion exchanges method is based on
Text Solution
|
- The radiocative lanthanide is
Text Solution
|
- Complete the following reaction
Text Solution
|
- The pair of lanthanides with the highest third ionization energy is
Text Solution
|
- All actinods have high desities except
Text Solution
|
- Which one of the following is an electronic configuration of thorium?
Text Solution
|
- Consider the following statement, (I) The size of the lanthanide M^(...
Text Solution
|
- The actinoids exhibit, more member of oxidation states in general than...
Text Solution
|
- Which of the following factor may be regarded as the main cause of lan...
Text Solution
|
- In which 5f subshell is half-filled?
Text Solution
|
- The actinides showing +7 oxidation state are:
Text Solution
|
- Which of the following is not an actinide?
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- Which of the following elements shows maximum number of different oxid...
Text Solution
|
- Gadolinium (Gd) has 4f^(7) 5 d^(1) 6s^(2) electronic configuration out...
Text Solution
|
- Which of the following elements is not an actinide?
Text Solution
|
- Which of the following graphs shown correct trends in the size of + 3 ...
Text Solution
|