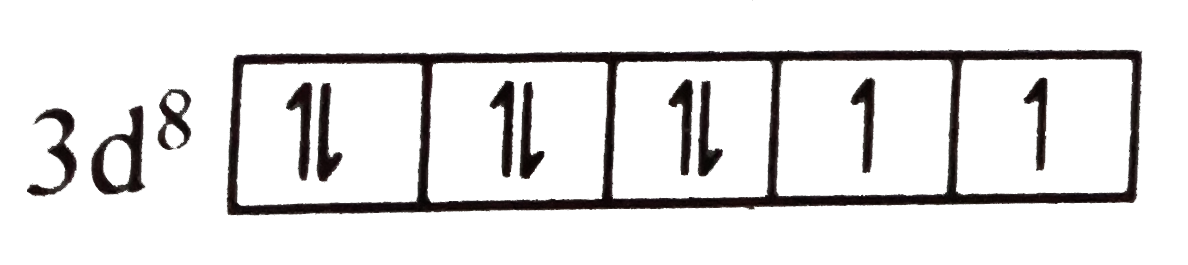A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-THE D AND F BLOCK ELEMENTS-AIPMT/NEET Questions
- The aqueous solution containing which one of the following ions will b...
Text Solution
|
- The main reason for larger number of oxidation state exhibited by the ...
Text Solution
|
- Which of the following pairs is coloured in aqueous solution?
Text Solution
|
- Which one of the elements with the following outer orbital configurati...
Text Solution
|
- which of the following electronts is present as the impurity to the m...
Text Solution
|
- For the four successive transition elements (Cr, Mn, Fe, and Co), the ...
Text Solution
|
- Identify the alloy containing a non metal as a constitunt in it
Text Solution
|
- Red precipitae is obtained when ethanol solution of dimethylglyoxime i...
Text Solution
|
- Four successive members of the first series of transition metals are l...
Text Solution
|
- KMnO4 can be prepared fromK2MnO4 as per the reaction: The reaction ...
Text Solution
|
- Which of the following statements about the interstitial compounds is ...
Text Solution
|
- The pair of compounds that can exist together is:
Text Solution
|
- Magnetic moment 2.83 BM is shown by which of the following ions?
Text Solution
|
- Reason of lanthanide contraction is
Text Solution
|
- Because of lanthnoid contraction, which of the following pairs of elem...
Text Solution
|
- Gadolinium belongsd to 4f series. It's atomic number is 64. which of t...
Text Solution
|
- Assuming complete ionization, same moles of which of the following com...
Text Solution
|
- Which is the correct order of increasing energy of the listed orbitals...
Text Solution
|
- Which one of the following statement is correct when SO(2) is passed ...
Text Solution
|
- The electronic configuration of Eu (Atomic No. 63), Gd (Atomic No. 64)...
Text Solution
|